It is estimated that more than 10 million people in the United States are affected by peripheral arterial disease (PAD) ...
Atherectomy Devices
This channel includes news and new technology innovations for atherectomy systems used in peripheral or coronary arteries to debulk lesions and vessel preparation prior to angioiplasty or stenting.
November 3, 2015 — Cardiovascular Systems Inc. (CSI) announced last week that the first two patients have been enrolled ...
July 22, 2015 — Cardiovascular Systems Inc. announced that it has received U.S. Food and Drug Administration (FDA) ...
May 12, 2015 — Cardiovascular Systems Inc. (CSI) featured two-year data from its ORBIT II study of the company’s ...
April 24, 2015 — Cardiovascular Systems Inc. announced that it has received U.S. Food and Drug Administration (FDA) ...
March 10, 2015 —George Adams, M.D., an interventional cardiologist with North Carolina Heart and Vascular, successfully ...
February 25, 2015 — CBSET, a not-for-profit preclinical research institute, announced that its scientists have defined ...
February 9, 2015 — Ensuring that patients with peripheral arterial disease (PAD) get tested and treated with minimally ...
Nov. 14, 2014 — Covidien releases 12-month results of the DEFINITIVE AR study, the first randomized study designed to ...
Nov. 6, 2014 — Cardiovascular Systems Inc. received the CE Mark for its Stealth 360º Orbital Atherectomy System (OAS) ...
Nov. 5, 2014 — Food and Drug Administration gave 510(k) clearance for the HawkOne directional atherectomy system. The ...
October 30, 2014 — Cardiovascular Systems Inc. announced that the first seven patients in Japan have been enrolled in ...
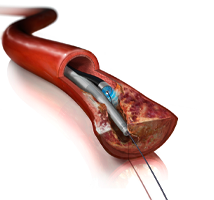
September 26, 2014 — The first large prospective study to examine the effectiveness of laser atherectomy in the ...

September 8, 2014 — Directional atherectomy is safe and effective as a frontline therapy for the treatment of peripheral ...

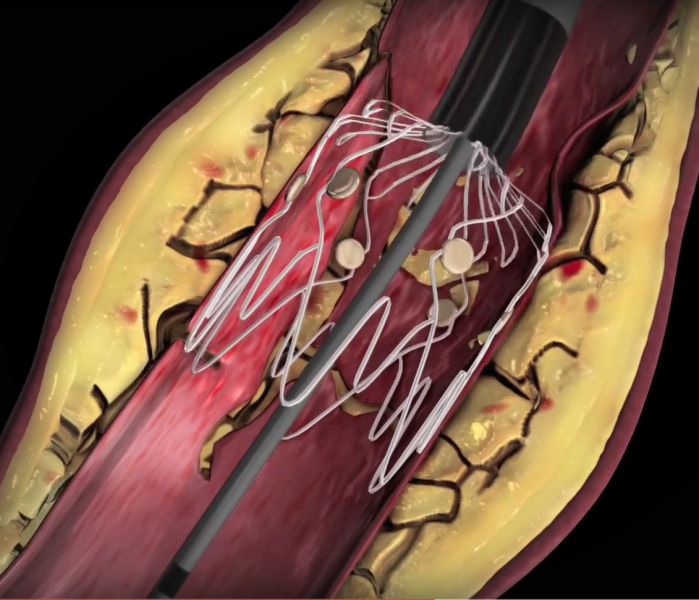
 February 22, 2016
February 22, 2016