July 11, 2008 - AngioScore Inc. today announced the launch of new longer and larger AngioSculpt PTA Scoring Balloon ...
July 11, 2008 - Micrus Endovascular Corp. said this week it received approval from the Ministry of Health, Labour and Welfare (MHLW) to market in Japan the Cerecyte microcoil product line, including the MicruSphere, Presidio, HeliPaq and UltiPaq Cerecyte embolic coils, for the endovascular treatment of cerebral aneurysms.
Bioheart Inc. offers the Bioheart 3370 Heart Failure Monitor, an interactive and simple-to-use at-home device designed to better monitor patients outside hospitals who are suffering from heart failure. The device, manufactured by RTX Healthcare A/S of Denmark, has FDA 510(k) market clearance and CE Mark in Europe.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 11, 2008 - St. Jude Medical Inc. today received European CE Mark approval and began its European launch of its line ...
July 11, 2008 - Bioheart Inc. this week said it secured worldwide, nonexclusive distribution rights to the Bioheart 3370 ...

Safe delivery of the balloon or stent through fragile vasculature during percutaneous transluminal coronary angioplasty (PTCA) procedures is an important criterion for successful patient outcomes.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Cardiology fellows are expanding their knowledge in cardiovascular imaging. Not only do virtually all cardiology fellows get Level 2 training in echocardiography and many in nuclear cardiology but now, an increasing number of fellows are training in cardiac MR and cardiac CT.
July 10, 2008 - The Senate overcame partisan gridlock on July 9, 2008, and passed the Medicare Improvements for Patients ...
What makes cardiologists more capable of reading cardiac imaging exams than other physicians such as radiologists, who ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

(Editor's note: this article was updated in March 2017 with links at the bottom to more current stent technology ...

Physicians recognize the importance of ventricular assist devices (VADs) every time a congestive heart failure (CHF) ...
July 10, 2008 - Boston Scientific Corp. this week launched its Atlantis .018 Peripheral Imaging Catheter, which reportedly brings existing 40 MHz coronary standard ultrasound imaging resolution to peripheral interventions. The company said the product is available for immediately release in the U.S.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
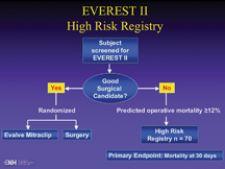
Preliminary results of a multicenter study show that the use of tiny clips to repair the mitral valve may someday ...
July 10, 2008 - Datascope Corp. said today it exercised its option to acquire the Peripheral Vascular Stent business of ...
The only safe assumption to make about today’s current gold standard imaging modality used to identify and evaluate atheromatous plaque in the peripheral arteries is that there is not one gold standard. To locate and evaluate these plaques that cause peripheral vascular disease (PVD), physicians have multiple tried-and-true imaging tests at their disposal.

 July 10, 2008
July 10, 2008



