May 12, 2010 – A new feature of a magnetic navigation platform facilitates remote manipulation of diagnostic devices used during electrophysiology (EP) procedures. Stereotaxis Inc. is introducing the Vdrive this week at the Heart Rhythm Society annual meeting. The device enables the electrophysiologist to fully and remotely control diagnostic catheters and accessories.
May 12, 2010 – A paclitaxel-eluting coronary stent received CE mark approval for a specific indication to treat diabetic patients. The Taxus Element incorporates a platinum chromium alloy and a new stent design. The Boston Scientific plans to launch the stent in June in the European Union and other CE mark countries.

A quiet revolution is going on in the world of patient risk stratification. It is becoming increasingly apparent that, while sudden cardiac death (SCD) manifests in the heart, it is triggered by the brain via the autonomic nervous system (ANS). Techniques taking this into account have the potential to be far more accurate and robust than other existing methods.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
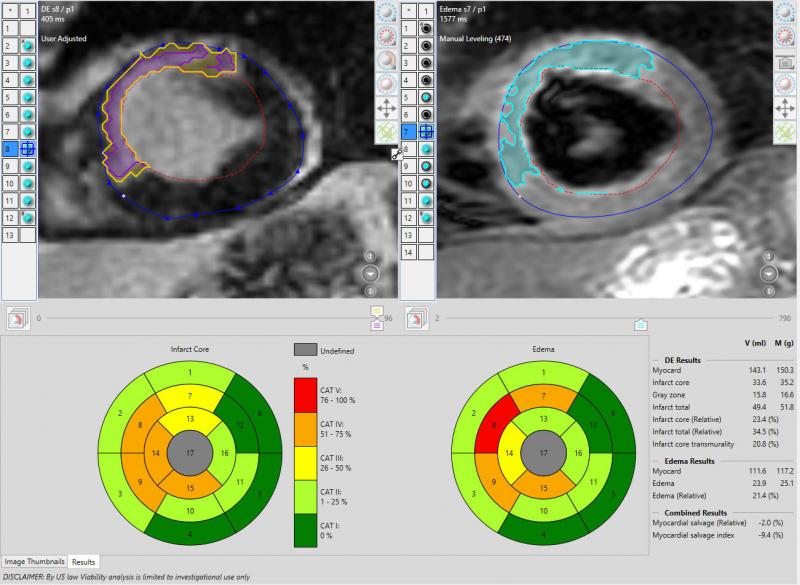
The importance of dealing with the epidemic of coronary artery disease (CAD) is well known, and the tools at our disposal to accurately recognize and manage it are evolving in very positive ways.

Early defibrillation is often referred to as the “critical link in the chain of survival,” according to the American Heart Association (AHA). But even in a hospital setting, the time from when a person suffers cardiac arrest to when they undergo defibrillation varies widely.
Atherectomy is a key treatment option in peripheral artery disease (PAD), and several physicians Diagnostic & Invasive Cardiology recently spoke with are fans of the laser over mechanical systems in most PAD cases. They say it is easier to use and eliminates the need for an embolic protection device, but it does have limitations when treating calcified lesions.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

New U.S. Food and Drug Administration (FDA) indications for two key heart failure device therapies may help improve survival, slow the progress of the disease and, in some cases, reverse its progression.
Monitoring congestive heart failure patients for any worsening in their condition is based mainly on patient compliance to accurately weigh themselves every day and report any unusual symptoms.

There were many unique technologies showcased at the recent American College of Cardiology (ACC) 2010 Scientific Sessions, which may impact the practice of cardiology in the near future. Voice Recognition, Structured Reporting
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 12, 2010 - Increased detection and treatment of abdominal aortic aneurysms (AAAs) and peripheral arterial disease (PAD) will drive the U.S. peripheral vascular device market to over $5.3 billion by 2016, according to a new report by iData Research.
May 11, 2010 - “Echocardiographic quantification is crucial in the diagnosis and management of patients with acquired and congenital heart disease,” said Leo Lopez, M.D., FASE, of Children’s Hospital at Montefiore, Bronx, N.Y., in a report published in the May issue of the Journal of the American Society of Echocardiography (JASE).

As intravascular ultrasound (IVUS) becomes a more common tool in cath labs to assess lesions and stent treatments, new innovations loom on the horizon. These tools include forward-looking IVUS and a combination IVUS/balloon catheter.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 11, 2010 – A cellular accessory has been added to an implantable cardiac device remote monitoring system to make it easier for patients to securely send information to their physicians. The Medtronic M-Link allows transmission of information via the CareLink Network using cellular signals, rather than a telephone landline.
May 11, 2010 – A wireless USB adaptor allows patients with implantable cardiac devices to stay connected with physicians without the need for a standard landline phone connection. Today St. Jude Medical launched its Wireless USB Adaptor for the Merlin@home electrophysiology (EP) device transmitter.
May 11, 2010 – An new iPad application integrates with and enhances the reporting and workflow management capabilities of a Web-based electrocardiogram (ECG) application. LifeWatch Services Inc. today launched the remote ECG report TeleViewer application for the Apple iPad.

 May 12, 2010
May 12, 2010








