March 21, 2011 - A pilot study in healthy children and adolescents shows that it is feasible to screen for undiagnosed heart conditions that increase the risk of sudden cardiac arrest (SCA). Adding a 10-minute electrocardiogram (ECG) to a history and physical examination identified unsuspected cases of potentially serious heart conditions.

For more than 20 years, radiation oncologists have emphasized the importance of radiation-tolerant heart rhythm devices for use in patients who require cardiovascular regulation. In the last three years, interest has increased, as medical physicists responded to the U.S. Food and Drug Administration (FDA) to promote investigational research.
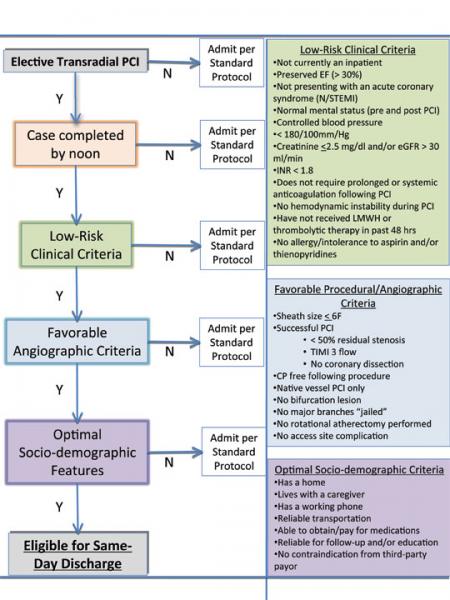
With the increased use of transradial cardiac catheterization throughout hospitals across the country, several results have become apparent. Transradial catheterization has been shown to have fewer bleeding complications, faster recovery time and improved patient satisfaction. It also offers a unique opportunity to improve resource utilization.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Transradial Approach (Left vs. Right) and Procedural Times During Coronary Procedures: TALENT study Alessandro Sciahbasi, et al. American Heart Journal, 2011, 161: 172-9 Study design: Prospective, randomized 1,467 patients (Jan. 2009-Dec. 2009) to right radial approach (RRA) or left radial approach (LRA).

Radial artery occlusion rate during transradial access procedures is strongly related to sheath and catheter size. Although worldwide most experienced practitioners (90 percent) use 5 and 6 French systems(1), high-quality coronary artery opacification can be obtained with 4 French systems.

Heart disease remains the number one killer of both men and women in the United States, with ST elevation myocardial infarction (STEMI) remaining one of the most feared manifestations. The underlying mechanism of a STEMI is plaque rupture and subsequent thrombosis of a coronary artery, causing subsequent myocardial ischemia, which progresses on to myocardial necrosis and infarction.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Tiny guide wires are designed to navigate vessels to reach a lesion or vessel segment. Once the tip of the device arrives at its destination, it acts as a guide that larger catheters can rapidly follow for easier delivery to the treatment site.
Two big advances in cardiac advanced visualization software were highlighted in December at the 2010 Radiological Society of North America (RSNA) meeting in Chicago. TeraRecon highlighted a computer-aided detection (CAD) software module to detect coronary artery stenosis.

Converting from paper-based electrocardiogram (ECG) files and reports to an electronic record system, or switching to a new electronic system, can improve workflow efficiency and speed the process of getting patients their test results. However, as with most health IT solutions, experts say there are no “out of the box” solutions, and every system will have interoperability bumps in the road.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Over the last 10 years, hospitals have phased out analog image intensifiers for flat-panel, digital detector angiography systems in their cath labs. As hospitals look to purchase current-generation digital angiography systems, buyers should be aware of several new technologies. Better Navigation Aids
In January the U.S. Food and Drug Administration (FDA) approved a big modifcation to the CoreValve U.S. pivotal clinical trial — eliminating the medical management arm of the study.
Computed tomography (CT) technology has developed tremendously in the past 30 years. Significant improvements in spatial and temporal resolution of current scanners allow for acquisition of high-resolution images of the small and fast-moving coronary arteries.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 18, 2011 – A clinical trial testing a robotic system to remotely deliver and manipulating coronary guide wires has begun. The PRECISE study is testing the CorPath 200 System, from Corindus Vascular Robotics.
March 18, 2011 – Researchers have found a link between high blood pressure and a greater drop in average walking speeds in older adults, according to results from a new study. The drop seems to occur even in study participants whose high blood pressure is successfully treated.
March 18, 2011 – Circulating through the bloodstream of every human being is a rare and powerful type of cell, one that can actually create new blood vessels to bypass blockages that cause heart attacks and peripheral artery disease (PAD). Though everyone has these cells – called endothelial progenitor cells – they are often dysfunctional in people prone to vascular disease.

 March 21, 2011
March 21, 2011





