November 1, 2011 – Boston Scientific released the schedule of its major events and product-related clinical research for the Cardiovascular Research Foundation's (CRF) 23rd annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, scheduled for Nov. 7-11 in San Francisco. TCT is designed to provide new insights for physicians who treat patients with cardiovascular disease and other vascular health issues.
November 1, 2011 – Volcano Corp., a developer and manufacturer of intravascular diagnosis and therapy guidance tools, said revenues for the third quarter of 2011 increased 18 percent versus the third quarter of 2010.
November 1, 2011 – Boston Scientific introduced its CardioTeach iPad app, an industry-first, free educational resource to help healthcare professionals better educate patients and caregivers about therapy options related to cardiovascular and peripheral diseases, specifically atrial and ventricular arrhythmias, coronary artery disease, heart failure, heart rhythm disorders and peripheral vascular disease.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
November 1, 2011 – The Angiographic and Imaging Core Laboratories of the Clinical Trials Center (CTC) at the Cardiovascular Research Foundation (CRF) in New York have formed a collaborative alliance with the Angiographic Core Laboratory of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center (HFMP). Led by distinguished physicians Gregg W. Stone, Jeffrey J. Popma and Gary S. Mintz, the collaborative research alliance will be known as CRFiCOR.
November 1, 2011 – Corindus Vascular Robotics said leading experts in interventional cardiology will speak about their clinical experience using the CorPath 200 System at a symposium co-sponsored by Corindus Vascular Robotics and Philips Healthcare. The event, entitled "Robotic-Assisted PCI, User Experience in Daily Practice," will take place at 7 a.m Thursday, Nov. 10, 2011 at the Moscone Center, Room 112 at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting.
TeraRecon will be showcasing iNtuition Cloud, the company’s flagship iNtuition solution in the form of a Web-based service at RSNA 2011.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 31, 2011 –Lantheus Medical announced it has been awarded a three-year supplier contract with Premier Purchasing Partners, the group purchasing unit of the Premier healthcare alliance, for its radiopharmaceutical imaging agents.
October 31, 2011 – GE Healthcare's announced in Milan, Italy, that the Lombardia Ministry of Health (MoH) purchased 135 units of GE Healthcare's pocket-sized, ultrasound medical imaging device Vscan for use by general practitioners in the region. This effort by GE Healthcare and the Lombardia MoH is not only the first of its kind for Italy, but also unique to Europe.
October 31, 2011 – NordicNeuroLab (NNL), a developer of products for functional neuroimaging, announced the launch of its magnetic resonance (MR)-compatible liquid crystal display (LCD) display. Branded the NNL LCD Monitor, the monitor is intended for use in functional MR imaging, patient comfort and relaxation, and as procedural magnetic resonance imaging (MRI) display.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 31, 2011 – To ensure MR imaging is as efficient and safe as possible, Toshiba America Medical Systems has unveiled enhancements to its Vantage Titan MR (magnetic resonance) product line, including the first high-density 16-channel flexible coil system (works-in-progress). The new additions to the Vantage Titan MR systems are designed to make it easier for clinicians to complete high-quality exams and improve diagnostic efficiency.
October 31, 2011 — On Oct. 18, 2011, the TICH-BIOTRONIC Pacemaker Wireless Monitoring Services Centre was co-founded in China’s Tianjin Economic-Technological Development Area (TEDA) by TEDA International Cardiovascular Hospital (TICH) of Tianjin and Biotronik (Beijing) SE & Co. KG of Germany.
October 31, 2011 — American College of Cardiology (ACC) President David Holmes, M.D., FACC, commended a congressional panel that met recently to discuss the physician payment system. The briefing was orchestrated by Reps. Phil Roe and Allyson Schwartz.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
October 31, 2011 — A panel presentation at the American College of Surgeons' annual clinical congress detailed how the contemporary practice of vascular surgery has evolved during the Iraq and Afghanistan wars. This has resulted in superior outcomes for complex, combat-related vascular injuries, including increased rates of amputation-free survival following extremity wounds.
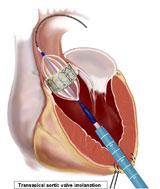
A better strategy to sell the costs of a transcatheter aortic valve implantation (TAVI) program to hospital administrators is not to base ROI on TAVI procedures, but on the increased surgical valve repair volume the program would create.

In a closely watched move – viewed as a big step toward a paradigm shift in how heart valves are repaired in the United States – a U.S. Food and Drug Administration (FDA) advisory panel in July recommended approval of the Edwards Lifesciences Sapien transcatheter heart valve.

 November 01, 2011
November 01, 2011













