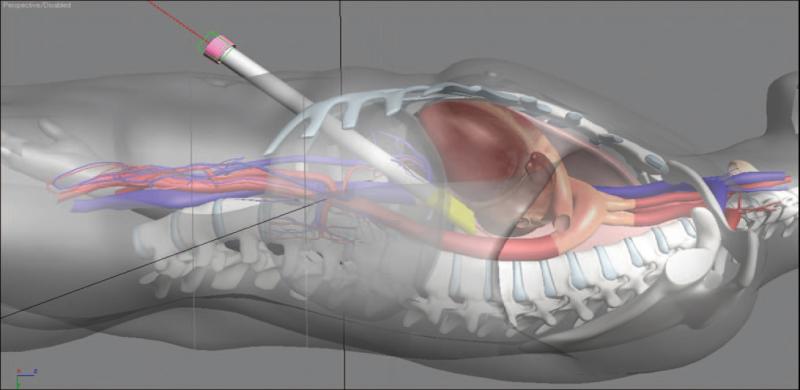
The ideal treatment of AF would be a truly minimally invasive procedure with results comparable to the Cox maze. This goal led to the convergence of surgical and electrophysiologic expertise to create a hybrid procedure for the treatment of symptomatic persistent AF.Using a pericardioscope, which is passed transabdominally through the central tendon of the diaphragm, the surgeon is able to directly visualize both atria, including the posterior left atrium and both sets of pulmonary vein.

Within the United States, the anticipated approval and launch of TAVR has received considerable attention, with speculation about who will perform the procedure. For example, many physicians are advocating a need for cardiac surgeons and interventional cardiologists to collaborate and work together as this technology becomes available in the United States.
In the past, when a patient suffered a prolonged cardiac or circulatory arrest during percutaneous coronary intervention (PCI) in the catheterization laboratory (cath lab) at Skane University Hospital–Lund, it was a catastrophic event. As any interventional cardiologist knows, the mortality rates in these situations are very high because it is essentially impossible to perform effective manual chest compressions while continuing PCI, especially in cases where prolonged resuscitation is required.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

At Memorial Hermann Heart and Vascular Institute – Texas Medical Center, we have expanded our already robust cardiac care offering to include radial approaches for interventional procedures. Offering radial approaches for interventional procedures has elevated Memorial Hermann’s level of patient care, by making interventions safer for patients by limiting complications.

Automated contrast media injectors control contrast dosage, record the amount used and speed injections to keep up with today’s fast computed tomography (CT) scanners. They also warn of potential hazards, such as air embolisms or extravasations.
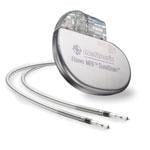
Estimates say up to 75 percent of patients with a pacemaker will need magnetic resonance imaging (MRI) over the course of their lifetime.[1] Yet as the industry has long been aware, MRIs can cause a number of adverse reactions when conducted on patients with a pacemaker. Aside from either the loss of pacing or inappropriate pacing, the MRI can cause current and heat to travel down to the heart muscle, resulting in local damage or life-threatening arrhythmias.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
There are currently three major trends in cardiac nuclear perfusion imaging technology: advances in detector hardware, improved software and new radiotracers. These advances have enhanced the diagnostic performance of both single photon emission computed tomography (SPECT) and positron emission tomography (PET) perfusion imaging.

Computed tomography (CT) scanner manufacturers say they are tracking numerous trends in the cardiac imaging market that are influencing their development efforts. Among them are concerns over ionizing radiation dose, increasing use of CT to evaluate chest pain patients, development of CT perfusion imaging and the need to simplify cardiac imaging and workflow.
November 22, 2011 — The thoracic aorta can pose major problems for endovascular repair when there are tortuous segments. Navigating an endograft through difficult territory can prevent its precise placement, especially in the aortic arch. At this year’s VEITH Symposium, Tilo Koelbel, M.D., Ph.D., described techniques that can be used in this situation.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 22, 2011 — The question remains whether patency rates for heparin-bonded polytetrafluoroethylene (PTFE) grafts are better than traditional PTFE bypasses; whether these types of grafts should be used in all lower extremity bypass situations is also a question.
November 22, 2011 — The controversial subject of “indiscriminate” stenting to treat asymptomatic carotid stenosis (ACS) was addressed at the 38th annual VEITH Symposium.
The use of 3-D/3-D registration from a syngo DynaCT Cardiac image and CT image was performed in syngo InSpace to help diagnose paravalvular mitral valve leakage. The imaging also served as procedural navigation aids.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
November 21, 2011 — Boston Scientific has reached an exclusive agreement with Catheter Robotics Inc. (CRI) to market the CRI Amigo system, a remote-controlled catheter system and related accessories. The two companies will focus their efforts in select European countries.
November 21, 2011 — Stereotaxis Inc. announced the launch of Odyssey Cinema Studio, a clinical procedure online broadcast platform. The system provides a turn-key solution for hospitals to facilitate high definition remote physician collaboration and global medical education.
November 21, 2011 — Micell Technologies Inc. announced the release of preliminary data from the first-in-human clinical study of the MiStent Sirolimus Drug Eluting Coronary Stent System (MiStent DES).

 November 22, 2011
November 22, 2011












