May 4, 2012 - CardioDex announces initial commercial use of a new femoral access site closure device. Recent data from commercial use of the device on 41 patients at St. Marien Hospital in Siegen, Germany showed 100% success rate and no complications.
May 4, 2012 - Edwards Lifesciences Corp. announced that new data from the European multi-center Triton trial studying the Edwards Intuity valve system highlights the promise of several important benefits, including the facilitation of small-incision surgery for aortic valve replacement (AVR), a high procedural success rate, and consistent and sustained hemodynamic valve performance at one year. The data were presented today during the Emerging Technologies and Techniques Forum at the American Association for Thoracic Surgery's 92nd annual meeting in San Francisco.
May 4, 2012 - Angioslide Ltd., a provider of embolic capture angioplasty solutions, announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its new 3x100 mm Proteus device for treating peripheral artery disease in below-the-knee (BTK) vasculature. The new 3/100 mm device accommodates 0.014-inch guidewires, which significantly broaden the potential use of Proteus technology.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
May 4, 2012 - A new balloon catheter system could advance the endovascular approach to treating obstructed arteries in the leg, offering an alternative to surgical revascularization. Peripheral artery disease affects about 12 to 14 percent of the general population. Revascularization can be achieved through bypass surgery or a number of minimally invasive endovascular techniques that seek to reduce or eliminate symptoms of reduced blood flow by improving tissue perfusion. Chronic total occlusions of the superficial femoral artery and popliteal artery, some of the most difficult lesions to recanalize with conventional guidewire techniques, were treated with this new system.
May 4, 2012 - An advanced, comprehensive cardiac monitoring system – PocketECG – combines and builds on the best features available from traditional Holter, event and mobile telemetry monitoring. Unlike other monitoring systems on the market, the recently introduced Spectocor PocketECG analyzes and then streams full-disclosure rhythm data to a monitoring center on a continuous basis.
May 4, 2012 - SMT Research & Development Ltd. (SMT) announced the addition of three key executives to its management team, including a new CEO, CFO and chief medical officer, plus two additions to its board of directors. The company also announced that its shareholders and board of directors have approved the company's name change to Keystone Heart Ltd.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
May 4, 2012 - The U.S. Food and Drug Administration (FDA) has approved the premarket approval application (PMA) for the Medinol Ltd. Presillion plus cobalt chromium (CoCr) coronary stent on a rapid exchange delivery system. This device is indicated for improving coronary luminal diameter in patients with symptomatic ischemic heart disease associated with stenotic lesions in de novo native coronary arteries (length < 30 mm) with a reference vessel diameter of 2.5 to 4 mm.
May 4, 2012 - Medtronic Inc. announced six-month pooled outcomes from randomized and crossover patients in the Symplicity HTN-2 clinical trial following renal denervation with the Symplicity renal denervation system showing significant, sustained blood pressure reduction in patients with treatment-resistant hypertension. These data presented at the European Society of Hypertension annual meeting showed patients (n=84) who received renal denervation treatment with Symplicity experienced a mean blood pressure reduction of -28/-10 mm Hg (p<0.001) at six months following treatment compared with baseline. No evidence of renal impairment was observed and renal function measures remained unchanged.
May 2, 2012 — The Spectranetics Corp. announced U.S. Food and Drug Administration (FDA) approval of the new advanced GlideLight Laser Sheath for removal of cardiac leads.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 2, 2012 — St. Jude Medical Inc. yesterday released its biannual product performance report (PPR) on its website, updating the performance of all the company’s cardiac rhythm management devices, including actively-monitored registry data that supports the safety and reliability of the current-generation Durata lead with Optim insulation. The report also provides a new, expanded analysis specifically addressing the performance of the company’s high-voltage leads.
May 2, 2012 — Vessix Vascular Inc. announced this week that it has received CE mark approval for its V2 Renal Denervation System for the treatment of hypertension. Renal denervation is a percutaneous catheter-based therapy that uses RF energy to disrupt renal sympathetic nerves whose hyperactivity leads to uncontrolled high blood pressure.
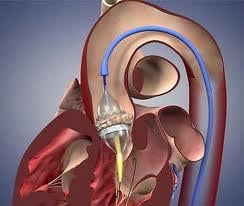
May 1, 2012 – The Centers for Medicare and Medicaid Services (CMS) announced today it will now cover transcatheter aortic valve replacement (TAVR) for Medicare patients under certain conditions. The coverage decision announced by CMS Acting Administrator Marilyn Tavenner offers important new technology to some of Medicare’s sickest patients.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 1, 2012 — Theragenics Corp. announced that Galt Medical, a wholly-owned subsidiary of Theragenics, has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its Galt VTI valved tearaway introducer.
May 1, 2012 — Cameron Health Inc. announced that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel voted 7-1 that sufficient data exists demonstrating the efficacy and safety of the S-ICD (subcutaneous-implantable cardioverter defibrillator) system for the treatment of sudden cardiac arrest (SCA).
May 1, 2012 - The use of a dedicated pediatric imaging department, with dedicated pediatric computed tomography (CT) technologists, for pediatric CT scans significantly reduces the radiation dose delivered to the patient, according to a study in the May issue of the Journal of the American College of Radiology.

 May 04, 2012
May 04, 2012












