Mercator MedSystems, a medical technology company whose platform Micro-Infusion Catheter technology announced preliminary results of its DANCE clinical trial that continues to follow patients at the University of California, San Francisco (UCSF) and San Francisco VA Medical Center. The technology offers catheter-guided, microfluid injection systems for site-specific, nonsystemic delivery of therapeutic agents directly across any peripheral or coronary blood vessel to treat peripheral vascular diseases, cancer, heart attacks, and other life-threatening diseases and disorders.
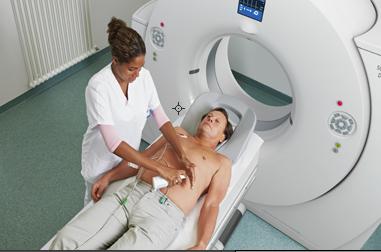
July 16, 2012 — The American College of Cardiology (ACC) and the American College of Radiology (ACR) are warning that the extreme cuts to funding for medical imaging scans in the 2013 Medicare Physician Fee Schedule Proposed Rule are unnecessary, unfounded and will undermine patient care.
July 16, 2012 — Reva Medical Inc. announced it completed clinical enrollment with the ReZolve drug-eluting bioresorbable scaffold. A total of 26 patients have been enrolled and there are no major adverse coronary events (MACE) reported to date.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
July 16, 2012 — LipoScience Inc., an in vitro diagnostic company developing clinical diagnostic tests using nuclear magnetic resonance (NMR) technology, announced publication of the findings of a clinical study in the current issue of the Journal of the American College of Cardiology indicating that high density lipoprotein (HDL) particle number has a stronger, more independent association with coronary heart disease and carotid atherosclerosis than HDL cholesterol (HDL-C).
July 16, 2012 — Medical imaging has always been an important part of clinical trials. Echocardiography, which uses sound waves to create moving real-time images of cardiac structure and function, provides an excellent noninvasive method to evaluate the impact of therapeutics on cardiac morphology.
July 16, 2012 — The National Heart, Lung, and Blood Institute (NHLBI) has awarded a $4.78 million grant to researchers at Cleveland Clinic’s Lerner Research Institute to use metabolomics — a new approach that focuses on the small-molecule byproducts of metabolism — for discovery of novel pathways linked to the development of cardiovascular diseases.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 16, 2012 — Cardiosolutions Inc., an early-stage company focused on the development of an innovative percutaneous system to treat patients with moderate to severe mitral valve regurgitation, today announced it has received a minority investment from Sorin Group. Sorin is a global medical device company and develops, manufactures, and markets medical technologies for cardiac surgery and for the treatment of cardiac rhythm disorders.
July 16, 2012 — The U.S. Food and Drug Administration (FDA) notified healthcare professionals of a Class I recall of Alere Triage products. Identified lots may have significantly decreased precision relative to the package insert, which could result in an increased frequency of false positive or false negative results.
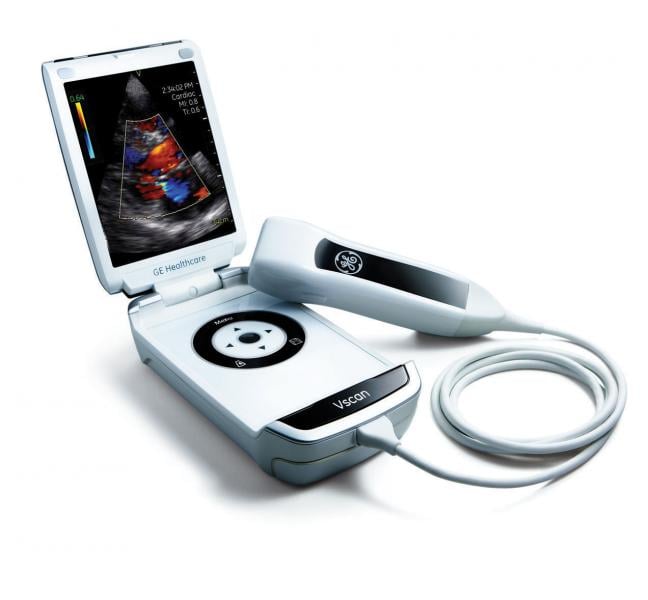
Echocardiography is a natural application for telemedicine, as handheld ultrasound devices and cloud-based transmission solutions make it possible to perform a test at a remote location and have consultation, in real time, by an expert thousands of miles away. Telemedicine can eliminate the need for travel, reduce wait time for test results, and is cost effective.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 13, 2012 — GE Healthcare and VPDiagnostics Inc. jointly announced today their co-marketing agreement for the VPDiagnostics’ MRI-PlaqueView software for carotid artery atherosclerosis analysis.
July 13, 2012 — Angioslide Ltd., a provider of embolic capture angioplasty solutions, today announced the successful deployment of a 3x100 mm Proteus device for treating peripheral artery disease (PAD) in below-the-knee (BTK) vasculature at Community Hospital in Munster, Ind.
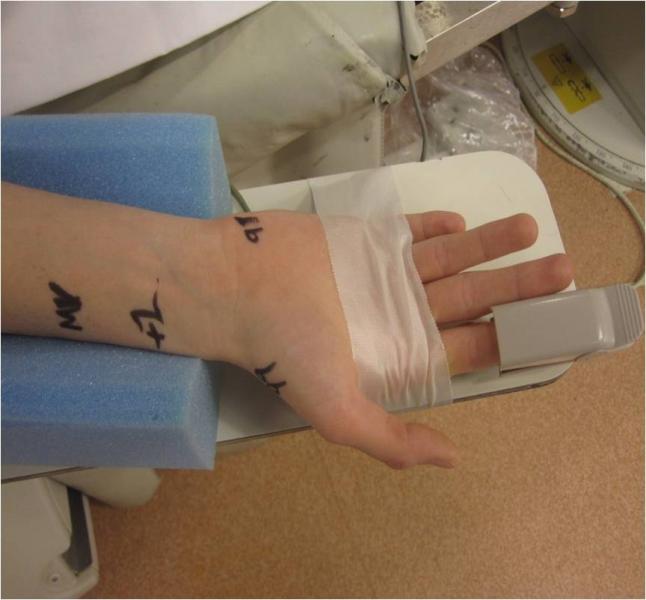
In the United States, radial artery catheterization is performed in the minority of diagnostic angiograms and cardiac stenting procedures despite the benefits it offers to patients in terms of reduced complications and faster mobility after the procedure. However, new data now suggests there might also be a cost savings. Research from the Perelman School of Medicine at the University of Pennsylvania, the University of Washington Medical Center, and the University of Pittsburgh School of Medicine, indicates that radial access may also offer a significant cost savings benefit to hospitals. The findings are published online first in Circulation: Cardiovascular Quality and Outcomes.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 13, 2012 — Royal Philips Electronics announced that it has embarked on a five-year joint research and development (R&D) program with the St. Petersburg State Polytechnical University, the Vavilov State Optical Institute and the Ioffe Physical Technical Institute.

An otherwise healthy pregnant woman who suffered cardiac arrest and was resuscitated, therapeutically cooled and then re-warmed, delivered a healthy, full-term baby 19 weeks later. Researchers published a unique case report online last week in Annals of Emergency Medicine.
July 12, 2012 — BioControl Medical has announced U.S. Food and Drug Administration (FDA) approval to begin the second phase of INOVATE-HF (Increase Of Vagal Tone in Heart Failure), a global, multi-center, investigational device exemption (IDE) clinical study of the company’s CardioFit system for heart failure.

 July 16, 2012
July 16, 2012








