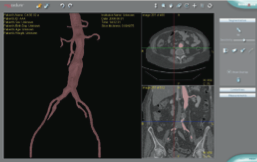
February 24, 2012 — Simbionix USA Corp. announced that it received U.S. Food and Drug Administration (FDA) clearance for the endovascular aneurysm repair (EVAR) application for the PROcedure Rehearsal Studio (PRS). Last year Simbionix received clearance for the PRS carotid intervention application.
The PROcedure rehearsal studio turns the patient's computed tomography (CT) scan into a 3-D visualization model. Simbionix has developed a technology to use this 3-D visualization model within its endovascular simulator, the Angio Mentor, to allow surgeons to evaluate endovascular surgical treatment options before surgery. PRS provides a 3-D model of the patient's vasculature and true-to-life vessel measurement tools.
After exporting the 3-D model into the Angio Mentor simulator practice environment, the physician is able to train and practice aneurysm repair on the patient's specific anatomy. For the first time, surgeons can practice endovascular abdominal aortic aneurysm repair, including precise deployment of the bifurcated and contralateral leg stent graft, deployment of iliac and aortic extensions and touch-up ballooning.
For more information: www.simbionix.com


 March 11, 2022
March 11, 2022 




