July 20, 2012 — The ability to see inside the arteries of vascular disease patients in high resolution before and during stenting procedures can offer valuable information. A new study of optical coherence tomography (OCT) in the Journal of Endovascular Therapy confirms the safety and feasibility of this imaging technique in the carotid arteries.
July 20, 2012 — Crux Biomedical announced it has received U.S. Food and Drug Administration (FDA) clearance for its novel inferior vena cava filter (VCF) with bi-directional retrieval.
July 20, 2012 —Acusphere Inc. announced that it had completed the marketing authorization application (MAA) dossier for its lead product candidate, Imagify (perflubutane polymer microspheres) for injectable suspension, and is now starting the process of filing the MAA dossier with the European Medicines Agency (EMA) to support the regulatory review of Imagify in the European Union.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
July 20, 2012 — Sony Electronics is announcing the world's first medical-grade monitor, model PVM-2551MD, based on organic light-emitting diode (OLED) technology. The new 25-inch monitor, which recently received U.S. Food and Drug Administration (FDA) 510(k) clearance, is expected to deliver significant benefits for a variety of surgical procedures and combines all the noted advantages of Sony's OLED technology: true-to-life color reproduction, high resolution and virtually no motion blur.

Each year, I find it interesting that some “new” technology being introduced by a vendor is actually an old, recycled idea.

An unusual exhibitor that seemed very out of place on the expo floor of the Heart Rhythm Society 2012 annual meeting was Ford Motor Company. Engineers, standing next to a brand new Ford sport utility vehicle, were there to get feedback from electrophysiologists on the use of car seats that can automatically monitor the driver’s electrocardiogram (ECG) whenever they are driving.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

The electrocardiogram (ECG) stress test remains one of the most widely used diagnostic tools for detecting coronary heart disease. While the basics of stress test systems have changed little since their adoption, technologies continue to evolve to improve waveform analysis as well as connectivity to electronic medical records (EMRs) and wireless data acquisition capabilities.
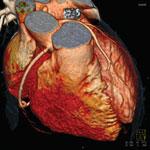
Coronary computed tomography angiography (CCTA) is a noninvasive tool that can be used for identifying myocardial ischemia and coronary artery plaques. It acts as a proven prognostic/diagnostic tool for patients who are asymptomatic as well as in the mild- to medium-risk spectrum of coronary artery disease (CAD).

Atherectomy devices are used in the cath lab to debulk lesions by cutting or laser ablating plaque, calcium and tissue hyperplasia from vessel walls, allowing recanalization of the vessel lumen as an end in itself, or in preparation for stenting. While some devices have an indication for the coronaries, the primary use of these devices is forperipheral artery disease (PAD) in the legs.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
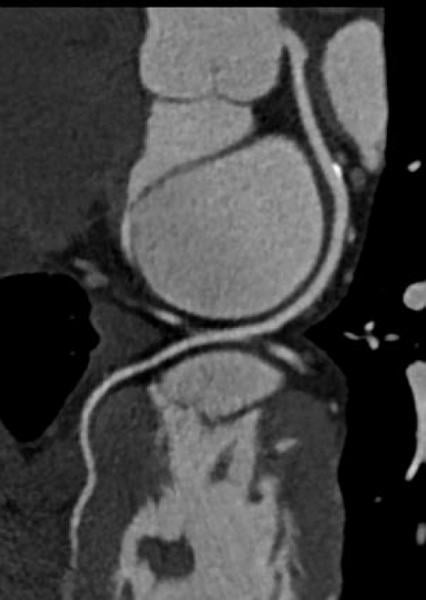
There are currently three major trends in cardiovascular computed tomography (CT) technology — lowering radiation dose, development of myocardial perfusion imaging and the use of CT datasets for procedural planning, especially for transcatheter aortic valve replacement (TAVR).

There is much discussion about the possibility of using transradial artery access to help reduce door-to-balloon times in ST-elevation myocardial infarction (STEMI) patients. Northside Hospital, Tampa Bay Heart Institute in St. Petersburg, Fla., tested this theory and found transradial helped lower door-to-balloon times and hospital length-of-stay.

Cath lab volumes have gone down in recent years for several reasons, but one of the biggest reasons might be market saturation. This is certainly the case in Chicago, where nearly every hospital has a cath lab, resulting in relatively low volumes at most centers, despite the fact they are located in the largest city in the Midwest.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 17, 2012 — Panasonic announced a new 3-D medical-grade 32-inch class monitor, the EJ-MDA32U-K. The new 3-D monitor is fully compliant with medical equipment standards, delivers exceptional 2-D and 3-D image quality, and can display multiple images from various sources at once. These combined features, paired with Panasonic’s renowned reliability, make the new monitor ideal for use in the surgical bubble.
July 17, 2012 — New analysis from Frost & Sullivan, “European Data Storage Market in Healthcare,” finds that the market earned revenues of $1,011.4 million in 2010 and estimates this to reach $2,473 million in 2017. The research covers hospital IT, research and imaging segments.

As the average age of the U.S. population and the number of patients at high risk for cardiovascular disease increase, the usage of cardio and endovascular stents continues to grow. Interventional cardiologists and registered nurses in the U.S. were recently asked about their perceptions and attitudes regarding current percutaneous coronary interventional (PCI) procedures and devices. They were also asked their stent brand and drug type preferences. In a statistically significant survey, they divulged which procedures and manufactures they preferred. This window into micro level stent usage offers informed insights into the overall market and reveals how dynamics between medical organizations and device manufacturers contribute to market trends.


 July 20, 2012
July 20, 2012








