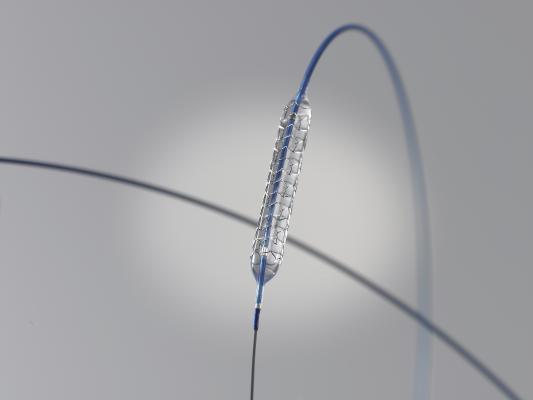
March 9, 2012 — Boston Scientific Corp. announced the launch of the Promus Element everolimus-eluting coronary stent system in Japan. The product was recently approved by the Ministry of Health, Labor and Welfare. It incorporates a platinum chromium (PtCr) alloy with an innovative stent design and an advanced catheter delivery system designed to provide physicians improved drug-eluting stent performance in treating patients with coronary artery disease. The company plans to begin marketing the product immediately in Japan.
"We are very pleased to launch the Promus Element stent system to Japanese physicians and their patients," said Yusuke Naiki, president of Boston Scientific Japan. "This everolimus-based stent system complements our broad coronary intervention portfolio and reinforces our global leadership in the drug-eluting stent market."
The Promus Element stent uses a proprietary PtCr alloy designed specifically for coronary stenting, which enables enhanced visibility, less recoil, excellent conformability and higher radial strength. It employs an advanced low-profile delivery system to enable precise stent placement across challenging lesions. The everolimus drug and fluorinated copolymer used have been studied in multiple randomized clinical trials and registries. The Promus Element Stent expands Boston Scientific's PtCr drug-eluting stent portfolio in Japan, which also includes the Taxus Element paclitaxel-eluting stent system.
"This approval marks two important milestones for Boston Scientific," said Hank Kucheman, CEO of Boston Scientific. "First, the Promus Element stent platform is now approved in every major market worldwide. Second, we have begun the last phase of our transition to higher margins on our everolimus stent offering as we shift to the internally manufactured Promus Element Stent. Boston Scientific remains the only company to offer physicians a choice of two proven drug and polymer combinations on an innovative coronary stent platform."
The company received CE mark approval for the Promus Element in 2009 and for the Promus Element Plus in 2011. In the United States, the Promus Element Plus was approved by the U.S. Food and Drug Administration (FDA) in 2011. The Taxus Element paclitaxel-eluting stent received Japan approval in 2011 and CE mark approval in 2010. It is commercialized in the United States. as the Ion paclitaxel-eluting stent, where it received FDA approval in 2011.


 July 02, 2024
July 02, 2024 









