July 13, 2012 — GE Healthcare and VPDiagnostics Inc. jointly announced today their co-marketing agreement for the VPDiagnostics’ MRI-PlaqueView software for carotid artery atherosclerosis analysis.
July 13, 2012 — Angioslide Ltd., a provider of embolic capture angioplasty solutions, today announced the successful deployment of a 3x100 mm Proteus device for treating peripheral artery disease (PAD) in below-the-knee (BTK) vasculature at Community Hospital in Munster, Ind.
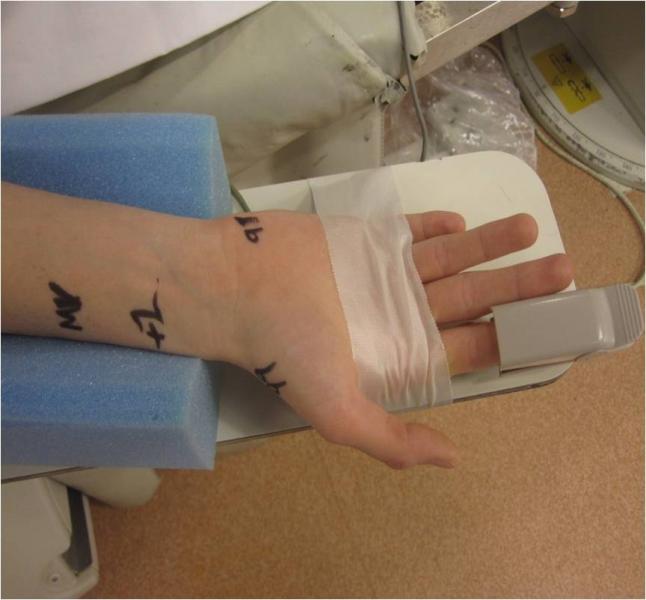
In the United States, radial artery catheterization is performed in the minority of diagnostic angiograms and cardiac stenting procedures despite the benefits it offers to patients in terms of reduced complications and faster mobility after the procedure. However, new data now suggests there might also be a cost savings. Research from the Perelman School of Medicine at the University of Pennsylvania, the University of Washington Medical Center, and the University of Pittsburgh School of Medicine, indicates that radial access may also offer a significant cost savings benefit to hospitals. The findings are published online first in Circulation: Cardiovascular Quality and Outcomes.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 13, 2012 — Royal Philips Electronics announced that it has embarked on a five-year joint research and development (R&D) program with the St. Petersburg State Polytechnical University, the Vavilov State Optical Institute and the Ioffe Physical Technical Institute.

An otherwise healthy pregnant woman who suffered cardiac arrest and was resuscitated, therapeutically cooled and then re-warmed, delivered a healthy, full-term baby 19 weeks later. Researchers published a unique case report online last week in Annals of Emergency Medicine.
July 12, 2012 — BioControl Medical has announced U.S. Food and Drug Administration (FDA) approval to begin the second phase of INOVATE-HF (Increase Of Vagal Tone in Heart Failure), a global, multi-center, investigational device exemption (IDE) clinical study of the company’s CardioFit system for heart failure.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Over the past decade there have been several advancements in prothrombin time (PT)/international normalized ration (INR) point-of-care testing (POCT) devices. This is usually performed at an outpatient Coumadin clinic or at the physician’s office, which can be inconvenient for patients, so there is a growing trend toward patient self-testing (PST) devices.
July 12, 2012 — Terumo Interventional Systems, a strategic business unit of Terumo Medical Corporation, a U.S. subsidiary of Terumo Corporation, announced the completion of U.S. patient enrollment in the Occlusive/StenoticPeripheral Artery Revascularization Study (OSPREY) designed to evaluate the safety and effectiveness of the Misago self-expanding stent system.
July 12, 2012 — Cardiac Science Corporation announced it will provide data from its Quinton Q-Tel Rehabilitation Management Systems to the American Association of Cardiovascular and Pulmonary Rehabilitation's (AACVPR) Outpatient Cardiac Rehabilitation Registry.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
July 11, 2012 — Ultrasonix Medical Corporation has received approval from the U.S. Food and Drug Administration (FDA) for its SonixGPS technology for vascular access procedures.
July 11, 2012 — Lantheus Medical Imaging Inc., a developer, manufacturer and distributor of diagnostic imaging agents, announced the publication of results from the CaRES (Contrast Echocardiography Registry for Safety Surveillance) multicenter safety registry for its ultrasound imaging agent Definity Vial for (Perflutren Lipid Microsphere) Injectable Suspension.
July 11, 2012 — Biotronik, a manufacturer of innovative medical technology, announced that enrollment is now complete for the much anticipated SPIRIT-ICD study, with 503 patients from 37 sites in 11 countries worldwide registered.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
July 11, 2012 — St. Jude Medical received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the ViewFlex Xtra intracardiac echocardiography (ICE) catheter.
July 10, 2012 — In CT (computed tomography) imaging, healthcare facilities strive to limit patient radiation dose while maintaining high image quality for accurate diagnoses. St. Elizabeth Healthcare, in Edgewood, Ky., is the first facility to be upgraded with Toshiba’s advanced dose reduction software, AIDR 3D, on its Aquilion ONE CT system.

July 10, 2012 — A study led by researchers at the University of California, San Francisco (UCSF) and the Group Health Research Institute (GHRI) shows that medical imaging is increasing even in health maintenance organization systems (HMOs), which don’t have a financial incentive to conduct them.


 July 13, 2012
July 13, 2012







