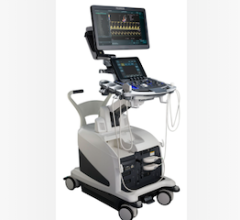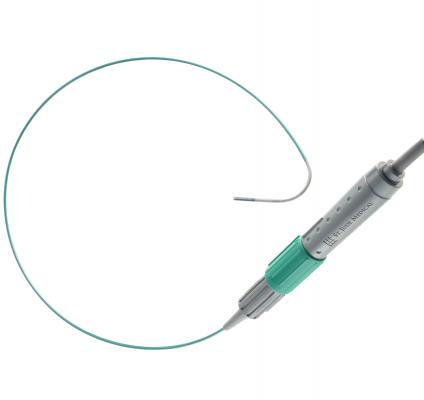
July 11, 2012 — St. Jude Medical received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the ViewFlex Xtra intracardiac echocardiography (ICE) catheter. The catheter technology is designed to be used with the ViewMate Z ultrasound console, a system that provides fast, clear images to help visualize cardiac structures and blood flow within the heart during complex electrophysiology or interventional procedures.
The ViewFlex ICE catheter has an ergonomic handle allowing for single-handed, four-way tip deflection and self-locking steering. The new handle helps streamline procedures by providing increased maneuverability with the potential to minimize catheter repositioning.
The images produced by the ViewFlex Xtra ICE catheter and the ViewMate Z ultrasound console help physicians confirm the location of cardiac structures and blood flow during procedures such as radiofrequency (RF) ablation to treat irregular heart rhythms and structural heart procedures to close atrial septal defects (a hole in the heart).
For more information: www.sjm.com


 October 09, 2025
October 09, 2025