The Medicare trustees have projected that the trust fund that finances Medicare’s hospital insurance coverage will remain solvent until 2026, two years beyond what was projected in last year’s report.

The U.S. Department of Health and Human Services (HHS) announced that more than half of all doctors and other eligible providers have received Medicare or Medicaid incentive payments for adopting or meaningfully using electronic health records (EHRs).
June 4, 2013 — During the 2013 Annual Meeting of the Society for Imaging Informatics in Medicine (SIIM), DR Systems will demonstrate its DR Systems Unity Enterprise Imaging Excellence Solution, e|HR for Meaningful Use and eMix, and electronic medical information exchange
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
HealthHelp’s MedTree ODS offers a transparent clinical decision support system that allows providers to self-govern the ordering process and receive approval for most decisions in less than one minute, 24 hours a day, seven days a week.
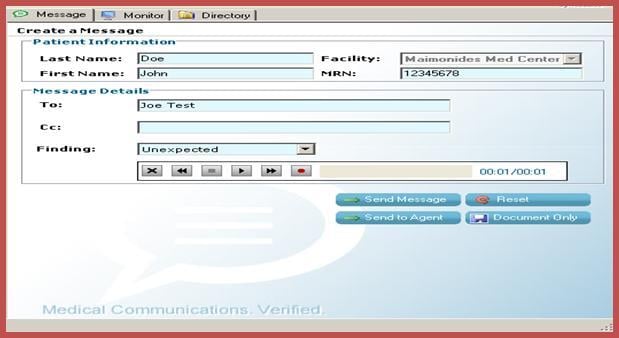
In today’s fast paced and “consumer-driven” medical environment, there are more demands imposed on clinicians to deliver quick and accurate care. These increasing demands have made it difficult for them to review imaging results in a timely fashion, resulting in potential dire consequences for their patients and medical-legal implications for their organizations.
Siemens Healthcare has announced that the U.S. Food and Drug Administration (FDA) recently cleared the company’s CAIPIRINHA (Controlled Aliasing in Volumetric Parallel Imaging Results IN Higher Acceleration) software as part of Siemens’ syngo MR D13A software package for parallel magnetic resonance (MR) imaging. The software helps enable patients with breath-holding difficulties to reduce the amount of time they hold their breath by up to 50 percent without sacrificing imaging resolution or contrast.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
ERT has introduced a centralized Ambulatory Blood Pressure Monitoring (ABPM) solution. ABPM enables biopharmaceutical companies to efficiently evaluate the efficacy and cardiac safety of both hypertensive and anti-hypertensive drugs as well as endpoints for cardiovascular and cerebrovascular outcomes.
The American College of Radiology supports the May 31, 2013 Government Accountability Office (GAO) report which calls for establishing minimum national standards and an oversight framework for accreditation of advanced diagnostic imaging services.
Telemed Solutions released its initial product, the TM-12, a PC-based wireless 12-lead electrocardiogram (ECG) system. It embeds a Bluetooth radio into an ECG acquisition device, then streams that ECG data from the patient to a standard Windows computer.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 3, 2013 — St. Jude Medical Inc. announced the company’s EnligHTN multi-electrode renal denervation system provides a safe, rapid and sustained reduction in blood pressure measurements after one year, as seen in data from the EnligHTN I trial that was presented at EuroPCR 2013 in Paris.

June 3, 2013 — Medtronic Inc. announced it has received CE mark for valve-in-valve (VIV) procedures using the CoreValve and CoreValve Evolut transcatheter aortic valve implantation (TAVI) systems in degenerated bioprosthetic surgical aortic valves.
June 3, 2013 — OrbusNeich has launched the world's first dual-therapy stent to address the challenges of delayed healing of the coronary artery associated with monotherapy drug-eluting stents (DES), the current standard of care for the treatment of coronary artery disease (CAD).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

May 31, 2013 — The American Society of Echocardiography (ASE) released a new expert consensus statement this week focusing on the practice of using ultrasound as a tool for improving patient physical examinations. “Focused Cardiac Ultrasound: Recommendations from the American Society of Echocardiography” will appear in the June issue of the Journal of the American Society of Echocardiography (JASE).
Digisonics will exhibit its Enterprise PACS and structured reporting solutions for cardiology, OB/GYN, radiology and general ultrasound studies at this year’s Society for Imaging Informatics in Medicine (SIIM) Annual Meeting.

The total U.S. market for PTCA and cutting balloon catheters was valued at $293.5 million in 2012. The PTCA balloon segment accounted for close to 90 percent of the total coronary balloon catheter market, with cutting balloons accounting for the remaining portion. The PTCA balloon catheter market is expected to increase over the next few years and the cutting balloon market is expected to experience a slight decrease.

 June 04, 2013
June 04, 2013











