At RSNA 2013, GE Healthcare will highlight the latest hardware and advanced applications for its Discovery IGS angiography platform. These updates are geared towards addressing the challenges of healthcare providers, support clinical decision making and helping clinicians in refining overall patient care.
Critical Diagnostics announced the clinical study, “Head-to-head comparison of two myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 vs. Galectin-3,” recently published online in Journal of the American College of Cardiology (JACC) comparing the company’s cardiac biomarker ST2 to Galectin-3 (Gal-3), a biomarker from BG Medicine, found ST2 to be superior.
Ascendian Healthcare Consulting announced a new service line to assist clients with ensuring the confidentiality, integrity and availability of patient data — the medical imaging client’s privacy, data security and patient safety consulting services.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
A study by Cutting Edge Information found that pharmaceutical companies measure cost per patient per month to allow trials of different sizes and durations to be compared easily. This measurement also helps to illustrate the high costs associated with delays.

The U.S. Senate passed legislation Sept. 26 to reauthorize, extend and improve operations of the Federal Helium Reserve in Texas, which will guarantee a stable supply of the gas used for cooling magnetic resonance imaging (MRI) systems.
CardioLogical Solutions announced that it has been issued two additional patents for its family of aortic embolic protection devices designed to prevent stroke and other ischemic complications. The new patents include its first issued patent for its Emboline CAP technology, the only aortic embolic protection solution to offer comprehensive arterial protection, as well as a further patent for its broad embolic deflector intellectual property portfolio. CardioLogical Solutions now has four issued patents for aortic embolic protection.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
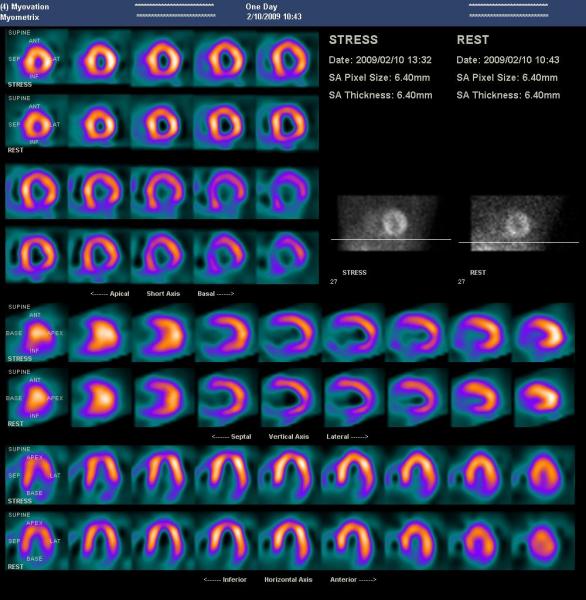
The highest levels of radiation dose in medical imaging are reported in nuclear medicine and pressure is growing for lower dose.
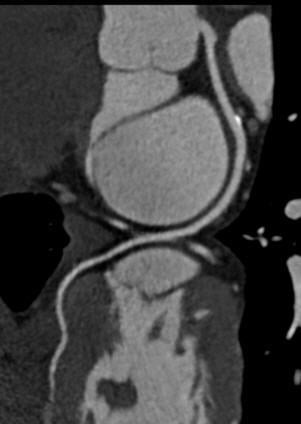
A large number of emergency room visits are for chest pain evaluation, and many hospitals are adopting CT angiography as a primary test to quickly rule-out coronary disease.
When leaders at Methodist Le Bonheur Healthcare (MLH), a not-for-profit health system based in Memphis, Tenn., saw the opportunity to standardize their suite of diagnostic imaging solutions, they capitalized on it. The seven-hospital system will deploy McKesson’s portfolio of enterprise medical imaging products across its various facilities to help improve physician collaboration, streamline data management and simplify its imaging archive process with a single point of access.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Patient enrollment has been initiated in a post-market registry for the COMBO Dual Therapy Stent to evaluate its long-term safety and performance in routine clinical practice. The prospective, multicenter, all-comers REMEDEE Registry (Multicenter, Prospective, Clinical Outcomes After Deployment of the Abluminal Sirolimus Coated Bio-Engineered Stent (Combo Bio-Engineered Sirolimus Eluting Stent) Post Market Registry) will enroll 1,000 patients at nine European sites. More than 100 patients have already been enrolled.
JenaValve Technology, Inc. announced that it has raised $62.5 million in a Series C venture round.
Rox Medical has crossed the halfway point in the CONTROL-HTN international, multi-center, randomized controlled hypertension trial. The milestone was met with the enrollment of the first patient from Prof. Markus van der Giet and Prof. Walter Zidek of the Charite Hospital, Benjamin Franklin Campus, Berlin. The CONTROL-HTN trial is evaluating the Rox Flow procedure — creating a small connection between artery and vein in the upper leg - for the treatment of resistant hypertension.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Parker Laboratories is introducing three, completely redesigned Thermasonic Gel Warmers at RSNA 2013. These include a single bottle gel warmer, a three-bottle gel warmer with preset temperature options and LED indicators, and a three-bottle gel warmer with LCD readout and temperature adjustment in one degree increments, in either Fahrenheit or Celsius.

In an effort to guide regulation of mobile medical apps, the U.S. Food and Drug Administration (FDA) issued a final guidance document for developers of mobile medical apps.

Medicare expenses for patients with acute myocardial infarction (AMI, heart attack) increased substantially between 1998 and 2008, with much of the increase coming in expenses 31 days or more after the patient was hospitalized, according to a study published by JAMA Internal Medicine, a JAMA Network publication.

 September 30, 2013
September 30, 2013