
In an expert consensus document e-published in Catheterization and Cardiovascular Interventions (CCI), the Society for Cardiovascular Angiography and Interventions (SCAI) offers recommendations for optimal use of three technologies that interventional cardiologists often use to assess the severity of blockages in heart arteries.
The American College of Cardiology (ACC) and the American Heart Association (AHA) released a clinical practice guideline to help primary care clinicians better identify adults who may be at high risk for developing atherosclerotic cardiovascular disease, stroke and who thus may benefit from lifestyle changes or drug therapy to help prevent it.
Resverlogix Corp. announced two additional results from the ongoing analysis of its Phase 2b ASSURE clinical trial using intravascular ultrasound (IVUS) to study high-risk cardiovascular disease (CVD) patients for assessing benefits of RVX-208.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
A study conducted in Ireland found that aspirin does not adequately protect 20 percent of patients with established heart disease.
Many healthcare systems are finding their general PACS and cardiology PACS are both aging and their image storage is reaching capacity. How do the diagnostic imaging departments address the needs of their cardiologists and radiologists?

The tiny device, about the size of the head of a pen, can be delivered via a delivery catheter through the venous system and placed directly into the myocardium of the right ventricle.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Neovasc Inc. announced that the first patent covering the company's innovative Tiara transcatheter mitral valve replacement technology has been issued by the U.S. Patent and Trademark Office. The new patent protects key aspects of the Tiara mitral valve prosthesis.
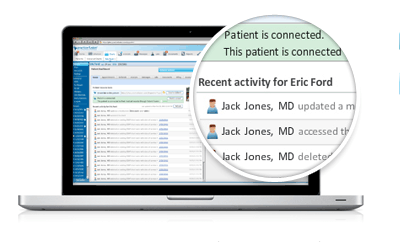
As health IT leaders make their way to New York for the New York eHealth Collaborative (NYeC) Digital Health Conference, Practice Fusion, the nation's largest healthcare platform, is looking at the key healthcare trend from 2013 and predicts what they might mean looking forward.
Professional cardiovascular societies and many working cardiologists question the U.S. Food and Drug Administration’s (FDA’s) recent recommendation that patients undergo genetic testing before taking Plavix (clopidogrel bisulfate).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
AngioScore, Inc., a developer of novel angioplasty catheters for use in the treatment of cardiovascular disease, announced detailed preliminary clinical trial results for the world’s first drug-coated scoring balloon catheter.
Clear Image Devices LLC (CiD) announced the release of its step platform to be used for standing patient ultrasound venous insufficiency studies. The Ultrasound Exam Steps enable sonographers to place the patient in the optimal position for safe, easy capture of the highest quality venous images of the upper and lower leg.
To help reduce the burden of cardiovascular disease, the nation's leading killer, New York-Presbyterian Hospital and Weill Cornell Medical College have created the Dalio Institute of Cardiovascular Imaging. Raymond T. Dalio, a life trustee of New York-Presbyterian Hospital, has made a gift of $20 million through his Dalio Foundation in support of the institute.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Eurozone economic slowdown has made it difficult for hospitals, particularly in Southern Europe, to procure new imaging modalities. With streamlined budgets and escalating number of medical procedures, the need to spend less on technologies while gaining the maximum benefit from them has sustained demand for high-quality refurbished imaging equipment.

The Society for Cardiovascular Angiography and Interventions (SCAI) published a first-of-its-kind paper defining best practices for the use of the transradial approach for diagnosing and treating blocked heart arteries.
InspireMD Inc., a developer of embolic protection stents, announced 12-month results from the MGuard for Acute ST Elevation Reperfusion (MASTER) trial demonstrating that the MGuard outperformed bare metal and drug-eluting stents in all-cause mortality in ST segment elevation myocardial infarction (STEMI) patients.

 November 18, 2013
November 18, 2013










