April 2, 2014 — At the 2014 annual scientific session of the American College of Cardiology (ACC), GE Healthcare introduced a new version of its Muse ECG (electrocardiograph) management system that now allows interoperability with non-GE ECG systems.

The key trial presented at the 2014 American College of Cardiology (ACC) that will likely be the one attendees will remember “they were there for” in the years to come was data showing transcatheter aortic valve replacement (TAVR) outperformed surgical valve replacement. The late-breaking presentation of the CoreValve U.S. pivotal high-risk trial showed the Medtronic self-expanding CoreValve device had a 26 percent survival benefit above the surgical valve implant patients.
April 2, 2014 — At the 63rd annual scientific session of the American College of Cardiology (ACC), Esaote premiered its new CrystaLine imaging technology that improves ultrasound’s diagnostic efficiency by enhancing image clarity and providing clinicians the ability to personalize image quality for each patient.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Statins are associated with a significant improvement in erectile function, a fact researchers hope will encourage men who need statins to reduce their risk of heart attack to take them, according to research to be presented at the American College of Cardiology’s 63rd Annual Scientific Session.
Acist Medical Systems Inc. announced at the American College of Cardiology's 63rd Annual Scientific Session in Washington, D.C., the global introduction of the new Acist|RXi Rapid Exchange FFR System — the world's first rapid exchange FFR system.
April 1, 2014 — Citing accelerating healthcare costs and finite resources, the American College of Cardiology (ACC) and the American Heart Association (AHA) announced they will begin incorporating value assessments into their clinical documents.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
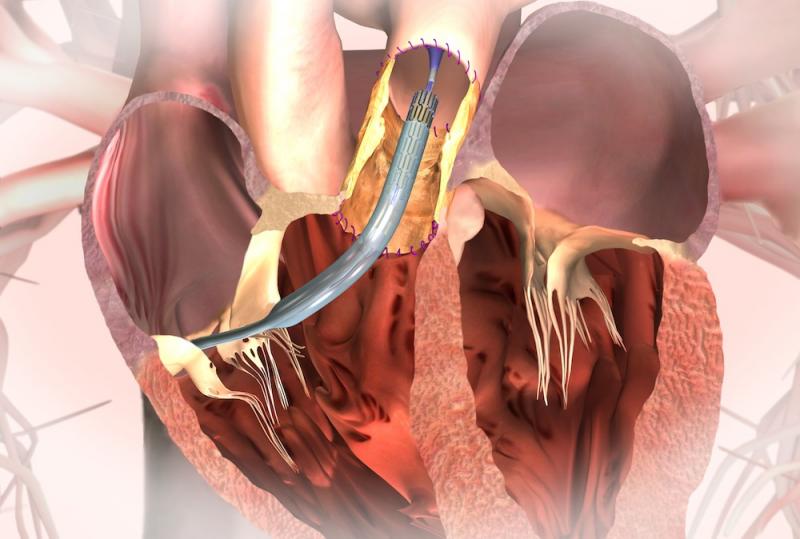
April 1, 2014 — Medtronic Inc. announced the one-year results of the Melody transcatheter pulmonary valve (TPV) U.S. post-approval study, which found that real-world use of the Melody TPV was associated with high procedural success, excellent valve function and few repeat procedures at the primary endpoint of six months. These results were sustained out to one year.
April 1, 2014 — GE Healthcare introduced the new Mac 2000 resting ECG (electrocardiography) system.
AccessClosure Inc. commercially launched itsMynx Ace vascular closure device, a safe and secure vascular closure product that provides consistent results with a new, easy-to-use deployment system to seal femoral artery access sites.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The Senate passed the Protecting Access to Medicare Act of 2014 (H.R. 4302) March 31, in a vote of 64 to 35.
The U.S. Food and Drug Administration (FDA) approved Abbott’s Supera Peripheral Stent System to treat people with blocked blood vessels in the upper leg caused by peripheral artery disease (PAD).
March 31, 2014 — BioCardia Inc. announced it received CE mark for its Morph AccessPro steerable introducer, designed for easier navigation through the vasculature during delivery of biotherapeutics and medical devices.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 31, 2014 — Biotronik announced new results from a sub-analysis of the TRUST trial, published in the European Heart Journal. This analysis examined recommended ICD (implantable cardioverter defibrillator) follow-up methods by comparing patient adherence in two groups: those who were given remote follow-ups with Biotronik Home Monitoring and those who had calendar-based, in-person follow-ups. Over the 15-month follow-up period, patient adherence was found to be 25 percent higher in the Home Monitoring group.

March 31, 2014 — Led by members of the American College of Cardiology (ACC), American Heart Association (AHA) and Society for Cardiovascular Angiography and Interventions (SCAI), 14 professional societies from across the world have developed a health policy statement that defines the clinical standards for structured reporting in the cardiac catheterization suite.

Privately held Arterial Remodeling Technologies (ART) signed a structured buyout agreement with Terumo Corp. Under the agreement, ART and Terumo Corp. will collaborate in the development of a drug eluting bioresorbable scaffold or stent, intended for use in the treatment of coronary artery disease.


 April 02, 2014
April 02, 2014











