AtriCure Inc. announced the first two patients in the Dual Epicardial and Endocardial Procedure (DEEP) clinical study have been enrolled and treated at PinnacleHealth CardioVascular Institute in Harrisburg, Pennsylvania. The study is the first of its kind in the United States for the treatment of persistent or longstanding persistent forms of atrial fibrillation (Afib).
Researchers at the University of North Carolina (UNC) School of Medicine have found the blood platelet protein Rasa3 is critical to the success of the common anti-platelet drug Plavix. Plavix breaks up blood clots during heart attacks and other arterial diseases.
Infraredx announced it will premiere the Advanced TVC Imaging System and the Muller NIRS-IVUS Catheter featuring Extended Bandwidth IVUS technology at the American College of Cardiology’s 2015 Annual Scientific Meeting in San Diego, March 14-16.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
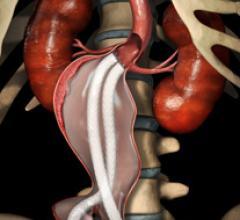
St.Vincent Heart Center is first in Indiana and second in the nation to participate in a clinical study for the Nellix EndoVascular Aneurysm Sealing System (EVAS), a surgical procedure designed to repair an abdominal aortic aneurysm (AAA).
Women veterans who had specialized heart tests were younger and more likely to be obese, depressed and suffer from post-traumatic stress disorder than male veterans, according to a study published in an American Heart Association journal.
A new study has found that high phosphate levels can cause a stress signal inside the cells that line blood vessels, leading to the release of microparticles that promote the formation of blood clots. The findings, which appear in an upcoming issue of the Journal of the American Society of Nephrology (JASN), provide new insights into how phosphate in the diet can impact heart health.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Toshiba America Medical Systems Inc.’s Dose Tracking System makes interventional procedures safer for patients by measuring peak radiation skin dose during the exam. To expand clinical applications, Toshiba is introducing enhancements to DTS for the entire Infinix product line, including the Elite, Select and Essential cardiovascular X-ray systems.
In a recent survey by the KLAS research firm, Siemens Healthcare was the designated vendor for healthcare providers in 47 percent of current partnership agreements. Moreover, the survey also found that Siemens is the vendor that more executives from the nation’s largest health systems would select over any other company as their first choice for a future partner.
Men with benign prostatic hyperplasia (BPH), have a new, breakthrough treatment option that is less invasive and has fewer complications than other minimally invasive treatments, according to research presented at the Society of Interventional Radiology's (SIR's) Annual Scientific Meeting.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
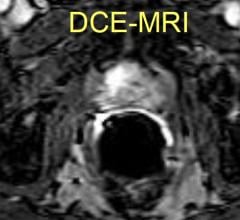
An innovative interventional radiology treatment has been found to offer chronic migraine sufferers sustained relief of their headaches, according to research being presented at the Society of Interventional Radiology's Annual Scientific Meeting.
Pablo Uceda, M.D., vascular surgeon with DFW Vascular, Dallas, Texas, was successful in using a new device to clear blood clots out of a patient's legs.
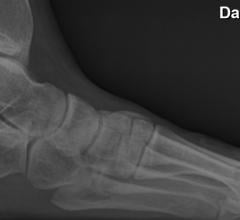
Patients suffering from chronic plantar fasciitis now have a new weapon against this debilitating foot ailment, according to research presented at the Society of Interventional Radiology's Annual Scientific Meeting.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
At one time, many children born with congenital heart disease (CHD) suffered from issues that carried fatal prognoses. Thanks to technological advancements in the past 30 to 40 years, Elizabeth Adams, M.D., of Penn State Hershey Children's Heart Group, can predict that a child born today has a 90 percent chance of living to adulthood, even with severe CHD.
The U.S. Food and Drug Administration (FDA) approved the VenaSeal closure system to permanently treat varicose veins of the legs by sealing the affected superficial veins using an adhesive agent.
Point of Care Anticoagulation software (PCDS AC) optimizes delivery of anticoagulation (AC) drugs and reduces adverse events associated with anticoagulation therapy.

 March 13, 2015
March 13, 2015