October 16, 2015 — Enrollment has successfully concluded in its multi-center, international CE mark clinical trials MEND ...

October 14, 2015 — Claret Medical, an innovator in cardiovascular cerebral protection, Data presented this week at ...

Results from a clinical trial showed that an everolimus-eluting bioresorbable vascular scaffold was non-inferior after one year compared to a current generation metallic drug-eluting stent (DES) in patients with coronary artery disease. Target lesion failure (TLF) was the primary endpoint for the study.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Edwards Lifesciences Corp. announced that details on the 13 first-in-human compassionate use cases with its Forma transcatheter tricuspid repair system were presented at the 27th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation. The results of the first seven cases were also published online this week in the Journal of the American College of Cardiology.
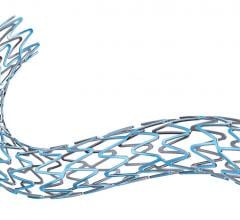
Two new advances in stent technology announced in recent days further reinforce the effectiveness of the next-generation bioabsorbable stents. Both new stents are designed to help reduce the risk of long-term complications for patients receiving a stent and will give interventional cardiologists more options in the care of cardiovascular patients.

One-year patient outcomes from the PARTNER II trial showed the low rate of 30-day complications with balloon-expandable transcatheter aortic valve replacement (TAVR) in high-risk and inoperable patients with aortic stenosis persisted with follow-up to one year.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 15, 2015 — U.S. Food and Drug Administration (FDA) has cleared Edwards Lifesciences Sapien XT transcatheter ...
Biotronik has announced results from the BIOSOLVE-II trial, investigating the safety and clinical performance of the world’s first clinically proven magnesium-based bioresorbable scaffold, at the Transcatheter Cardiovascular Therapeutics (TCT) 2015. The bioresorbable scaffold met its primary angiographic endpoint and demonstrated an outstanding safety profile. The announcement of clinical data from BIOSOLVE-II will be accompanied by publication in The Lancet.
C. R. Bard Inc. announced the presentation of the 12-month results from the Lutonix Global Real-World Registry at the Transcatheter Cardiovascular Therapeutics (TCT) 2015 meeting. These results come just months after publication in the New England Journal of Medicine of the one-year data from the Lutonix 035 drug coated balloon (DCB) catheter pivotal, randomized LEVANT 2 trial.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Tryton Medical Inc. announced results from the pivotal Tryton Confirmatory Study confirming the acceptable acute safety profile of the Tryton Side Branch Stent for the treatment of coronary bifurcation lesions in vessels appropriate for a ≥2.5mm stent. Results were presented as part of the Featured Clinical Research session at Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation (CRF) being held in San Francisco, California.
On Oct. 7, 2015, Cook Medical initiated a voluntary recall for select sizes of Beacon Tip Angiographic Catheters. This recall includes all lots of these select sizes of the Beacon Tip Angiographic Catheters. This recall is an expansion of the voluntary lot-specific recall issued on July 2, 2015.
AtriCure Inc. announced it has entered into a definitive merger agreement under which AtriCure has agreed to acquire nContact Inc., a privately held developer of innovative cardiac ablation solutions.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Boston Scientific Corp. announced it has closed on an additional round of financing with MValve Technologies Ltd., developer of a percutaneous mitral valve replacement system designed to work with the Boston Scientific Lotus Valve. Boston Scientific has provided the company with funding since 2012 and has an exclusive option to acquire MValve.
Kopp Development Inc. has released a new entryway system for magnetic resonance imaging (MRI) rooms — FerrAlert Halo II Plus. This detection system dramatically reduces alarm fatigue by not alarming on the MRI door and ferromagnetic objects exiting the MRI room. Alarm fatigue reduction is a top priority for ECRI and The Joint Commission.
The Lancet published results online that show Abbott’s ARCHITECT STAT High Sensitive Troponin-I (hsTnl) test may rule out myocardial infarction (MI) in two-thirds of very-low risk patients, allowing prompt discharge from A&E (Accident and Emergency). The study identified a precise new threshold of troponin of <5 ng/L which, together with other clinical factors, allows doctors to rule out heart attacks in the majority of patients, reducing prolonged waiting for monitoring with additional tests, unnecessary procedures and hospital admissions.

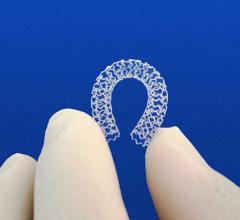
 October 16, 2015
October 16, 2015