Oct. 7, 2025 — Cagent Vascular has announced the commercial launch of the Serranator PTA Serration Balloon Catheter in 7.0 mm and 8.0 mm diameters. This expansion brings the proven benefits of ...
Angiographic Catheter
Oct. 7, 2025 — Cagent Vascular has announced the commercial launch of the Serranator PTA Serration Balloon Catheter in 7 ...
Sept. 29, 2025 — AngioSafe has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its ...
January 18, 2024 — Summa Therapeutics, LLC announced that the first-in-man injectable angioplasty procedures for ...
October 21, 2022 — Medtronic plc, a global leader in healthcare technology, today announced it has received U.S. Food ...
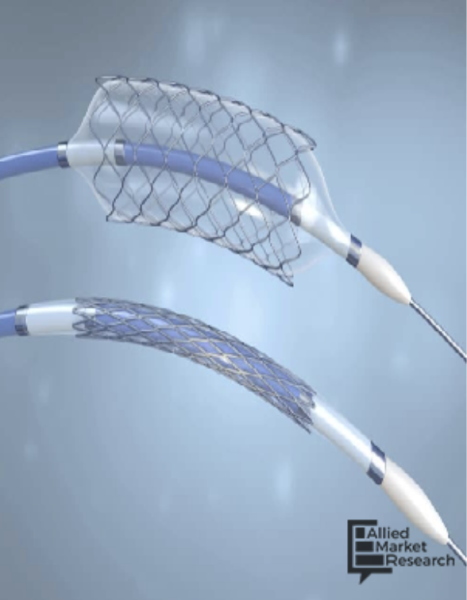
According to a new report from Allied Market Research, the global catheters market was valued at $22.7 billion in 2021 ...
April 7, 2020 — Boston Scientific Corp. is recalling its Imager II 5 French angiographic catheters, because there is a ...

Philips is working on a prototype cath lab angiographic imaging system that might be able to replace the current X-ray ...
October 9, 2019 — Medtronic is recalling the 6 French Sherpa NX Active Guide Catheter due to a risk of the outer ...
July 25, 2016 — Stryker Sustainability Solutions (formerly Ascent Healthcare Solutions) is recalling Angiodynamics Soft ...
October 14, 2015 — On Oct. 7, 2015, Cook Medical initiated a voluntary recall for select sizes of Beacon Tip ...
August 11, 2015 — Cook Medical initiated a voluntary recall July 2 of 2,239 lots of beacon tip angiographic catheters. A ...
The Mikro-Cath disposable blood pressure catheter delivers accurate and reliable hemodynamic data from within the heart ...
October 15, 2012 — Surefire Medical Inc. has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market ...
Aug. 8, 2012 — Vascular Solutions Inc. launched the SuperCross FT, a new flexible-tip version of its line of SuperCross ...






 October 08, 2025
October 08, 2025









