To meet both clinical and research needs of magnetic resonance (MR) customers, Toshiba America Medical Systems Inc. has implemented software and hardware upgrades for the Vantage Titan 3T MR imaging system. Available for new systems and for existing installations, these upgrades improve image quality and workflow so those in both the clinical and research settings have access to the highest levels of performance and information to provide the best possible care.
The Healthcare Information and Management Systems Society (HIMSS) submitted comments to the Department of Health and Human Services on the Meaningful Use Stage 3 proposed rule and the 2015 Edition Health IT Certification Criteria.
Oxford Instruments plc has acquired Medical Imaging Resources Inc. (MIR). MIR specializes in the build, lease and service of mobile medical imaging labs.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
CVRx Inc. announced that positive results from the 'Barostim Therapy for Heart Failure' randomized, controlled clinical trial were presented at the ESC-Heart Failure 2015 Annual Conference in a late-breaking trial session. Results were presented by Prof. Jochen Müller-Ehmsen, M.D., Ph.D., from Asklepios Hospital Altona in Hamburg, Germany.
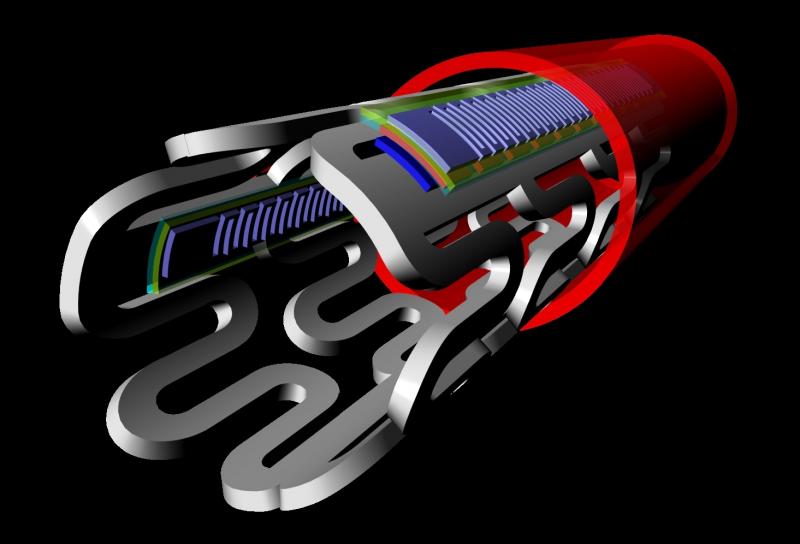
With the recent innovation of flexible microelectronic sensor circuits that can be made from bioresorbable materials, it is now possible to create “electronic stents” to monitor a treated lesion site inside a patient’s artery. A Korean-led team recently published their research on the first device of this kind, showing proof of concept in the American Chemical Society journal ACS Nano.[1]
The U.S. Food and Drug Administration (FDA) has cleared the InvisionECG technology from InvisionHeart. The mobile electrocardiogram (ECG) technology uses a secure cloud-based, healthcare IT platform to capture and manage 12-lead ECGs.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

A virtual-heart platform proposed by Stony Brook University researchers and colleagues has received funding from the National Science Foundation (NSF) in the amount of $4.2 million over five years. The platform was designed to improve and accelerate medical-device development and testing.
OrbusNeich has announced that the first U.S. patient has been enrolled in the HARMONEE (Harmonized Assessment by Randomized, Multi-center Study of OrbusNEich's COMBO StEnt) stent study. The study is being conducted under the framework of the joint Japan-U.S. Harmonization-By-Doing (HBD) initiative and will support the company's planned application for Shonin approval in Japan and to meet the feasibility trial requirements in the U.S.
Kareo announced the launch of its Apple Watch App. This most recent innovation extends the functionality of the company’s electronic health record (EHR) to Apple Watch, streamlining care delivery and enhancing the patient experience by improving communications, reducing patient wait times and increasing practice efficiency.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Medic Vision Imaging Solutions Ltd. announced the introduction and availability of SafeCT Dose Reporting. Healthcare facilities can now, for the first time, use a single software platform to meet their low dose computed tomography (CT) imaging needs while also conforming to CT dose monitoring and reporting standards.
A microsupercapacitor designed by scientists at Rice University that may find its way into personal and even wearable electronics is getting an upgrade. The laser-induced graphene device benefits greatly when boron becomes part of the mix.
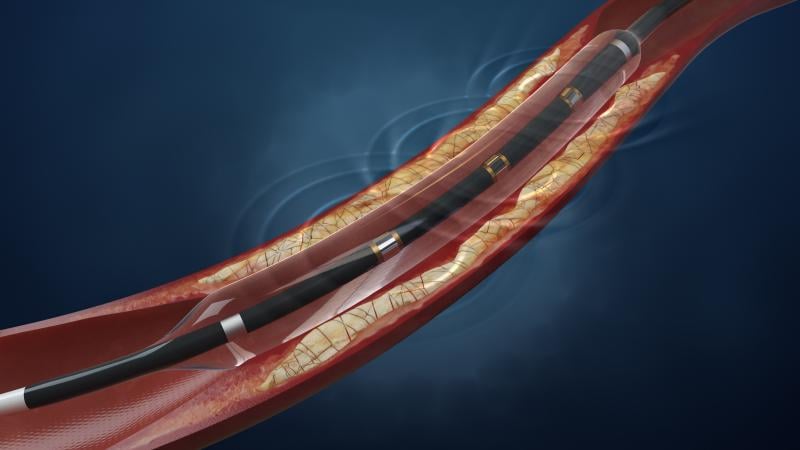
Shockwave Medical announced $40 million in funding, co-led by returning investor Sofinnova Partners and new investor Venrock. Also participating were RA Capital, Deerfield, Sectoral Asset Management, Ally Bridge Group and two undisclosed large-cap strategic investors.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Dassault Systèmes announced during its SIMULIA Community Conference that the first heart model from its “Living Heart Project” will be commercially available on May 29, 2015. Powered by Dassault Systèmes’ 3DEXPERIENCE platform’s realistic simulation applications, the commercial, high-fidelity, scientifically validated 3-D simulator of a four-chamber human heart is the first product of its kind. With this model, device manufacturers, researchers and medical professionals will be able to perform virtual tests and visualize the heart’s response in ways that are not possible with traditional physical testing.
A new study evaluating the Boston Scientific Lotus Valve System demonstrated an extremely low rate of paravalvular aortic regurgitation (leakage) for a transcatheter aortic replacement valve. Study data also showed a cardiovascular mortality rate of less than 2 percent at 30 days.

Privately-held company Arterial Remodeling Technologies (ART) announced CE Mark clearance for its next-generation drug-free, pure bioresorbable scaffold used to treat coronary artery disease. The CE Mark was achieved following the completion of extensive pre-clinical research — this included up to three-years of follow-up, and supportive clinical results from leading coronary angioplasty centres such as the Hôpital Européen Georges Pompidou in Paris and investigators such as Jean Fajadet, M.D., at the Clinique Pasteur in Toulouse.

 May 29, 2015
May 29, 2015












