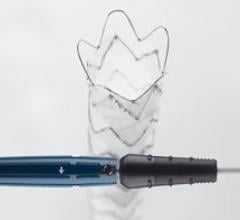
Medtronic plc announced the recent CE Mark and commercial launch for an expanded size matrix of the Resolute Onyx DES, a next-generation drug-eluting stent now available in 4.5 and 5 mm diameter sizes. The CE Mark also approved several new product indications including treatment of left main vessels and small vessels.
Abbott announced that it has acquired private medical device company Kalila Medical Inc. Kalila Medical is a developer of next-generation access technologies used in cardiac electrophysiology procedures for the treatment of heart rhythm disorders, including atrial fibrillation. Financial terms were not disclosed.
Agfa HealthCare announced it would be showcasing its convergence approach to enterprise imaging during the 2016 Healthcare Information and Management Systems Society annual conference (HIMSS16). The company’s Enterprise Imaging and Enterprise Content Management (ECM) platforms will be on display at the annual conference and exhibition, March 1 - 3, in Las Vegas.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
On February 1, the first day of American Heart Month, the American Heart Association (AHA) announced plans to develop a first-of-its-kind workplace health solution that leverages the cognitive computing power of IBM Watson.
The findings of a major study led by cardiovascular imaging specialists at Allegheny General Hospital (AGH) suggest magnetic resonance imaging (MRI) is a safe and effective diagnostic procedure for patients with implantable cardiac devices.
Alpha Source, a Milwaukee-based provider of healthcare technology management solutions, announced the publication of a retroactive data study on the total cost of ownership of ultrasound imaging equipment.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
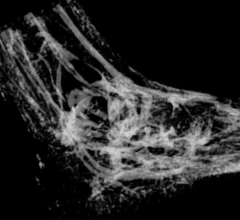
Diseased hearts may be thrown out of rhythm by structural differences, now visible for the first time, in protein groups that connect heart muscle cells, according to the authors of a study published in the journal Nature Communications online.
Intact Vascular Inc. announced that positive six-month results from its Tack Optimized Balloon Angioplasty – Below-the-Knee (TOBA-BTK) clinical study were presented at the LINC 2016 conference by principal investigator Marianne Brodmann, M.D.
IBM Watson Health announced that the 2015/2016 Best in KLAS: Software & Services report recognized two of its newly acquired companies for population health and imaging solutions.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Boston Scientific Corp. and Accenture have developed a cloud-based, data-driven digital health solution for hospitals to help improve patient outcomes and reduce costs to treat patients with chronic cardiovascular diseases.
In November, following the U.S. Food and Drug Administration approval of Cook Medical’s Zenith Alpha Thoracic Endovascular Graft, the first patient was treated with the device in the United States.
A new imaging technique could reduce the need for amputation in patients with critical limb ischemia (CLI), according to a study published in the scientific journal JACC.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Health division of Wolters Kluwer announced the release of a complimentary e-book that demonstrates the critical role of integrated clinical decision support (CDS) to driving value and optimizing patient care in today’s healthcare environments.
Effective Jan. 1, 2016, the American Medical Association (AMA) Current Procedural Terminology (CPT) Editorial Panel assigned a new category III CPT code, 0399T, for use in reporting myocardial strain imaging for the detection of myocardial deformation.
UltraSPECT Inc. has marked the 450th U.S. installation of its low-dose image processing technology for nuclear medicine. The milestone was reached as Firelands Regional Medical Center, Sandusky, Ohio, acquired Xpress3.Cardiac imaging solution to reduce radiation exposure for patients and staff and meet the American Society of Nuclear Cardiology (ASNC) dose-reduction guidelines.


 February 02, 2016
February 02, 2016