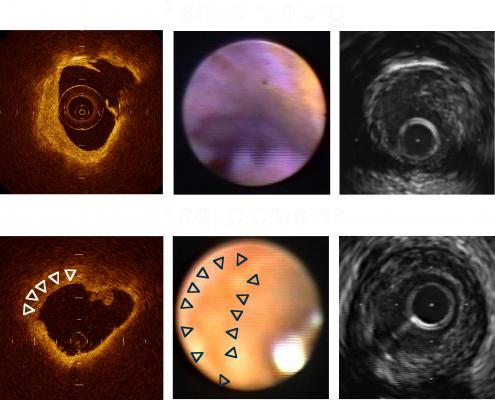
August 3, 2015 — Pie Medical Imaging announced the release of CAAS IntraVascular 2.0, the dedicated software for analysis of intravascular ultrasound (IVUS), near-infrared spectroscopy (NIRS)-IVUS and optical coherence tomography (OCT) datasets. This new release contains an optimized OCT analysis and the in-depth analysis of NIRS-IVUS datasets. Data can be imported easily from the console or picture archiving and communication system (PACS) into the study list, which provides a clear overview of your data. CAAS IntraVascular has U.S. Food and Drug Administration (FDA) 510(k) clearance for both IVUS and OCT.
The raw data import supports files of both OCT (St. Jude Medical Inc.) and OFDI (Terumo) format. Z-offset correction enables users to adjust the calibration, even after the export from the console. Data analysis is fast and easy with automatic lumen contour detection, EEM segmentation and stent strut analysis. The position of the struts is clearly visualized and automatically classified into covered, uncovered and malapposed struts. Also a 3-D visualization of the vessel and stent struts is provided.
Besides the current IVUS and NIRS-IVUS functionality of lumen, contour and stent contour detection, CAAS IntraVascular 2.0 contains an extensive in-depth Chemogram analysis module. This module provides visualization and dedicated analysis of NIRS-IVUS data such as custom LCBI measurements.
CAAS IntraVascular 2.0 contains on-the-fly reporting and results can also be exported easily in an XML file (Microsoft Excel-compatible).
For more information: www.piemedicalimaging.com

 May 12, 2020
May 12, 2020 









