New research finds that adding a digital health tool to traditional cardiac rehabilitation appears to help people recovering from a heart attack lose significantly more weight in a relatively short period of time.
A new study finds that despite increased understanding of heart disease risk factors and the need for preventive lifestyle changes, patients suffering the most severe type of heart attack have become younger and more obese.
March 31, 2016 — After a lengthy review of increasing medical evidence, the American Heart Association has published a ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Patients undergoing minimally invasive mitral valve repair or replacement (mini-MVR) have similar outcomes as patients undergoing conventional surgery, according to an article posted online by The Annals of Thoracic Surgery.
Digisonics will exhibit its latest functionality for cardiovascular information system (CVIS) solutions at the American College of Cardiology’s (ACC) 65th annual scientific session and expo, April 2-4 in Chicago.
The Smartdop XT by Koven is a fully automated vascular testing system that enables rapid, multiple-level bilateral studies in minutes.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Direct Flow Medical Inc. announced European commercial market launch of the DirecTrack Delivery System, a next-generation delivery system for the Direct Flow Medical Transcatheter Aortic Valve System.

Healthcare providers will need to start preparing for the next big healthcare information technology (IT) implementation ...
Toshiba America Medical Systems Inc. announced it would demonstrate the following diagnostic imaging technologies at this year’s American College of Cardiology 2016 Annual Scientific Session, April 2-4 in Chicago:
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Corindus Vascular Robotics Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared the CorPath System for use in peripheral vascular interventions.
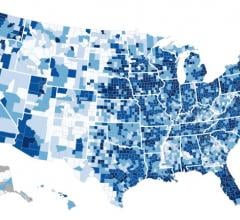
The Centers for Medicare & Medicaid Services Office of Minority Health (CMS OMH) released a new interactive map to increase understanding of geographic disparities in chronic disease among Medicare beneficiaries.
St. Jude Medical Inc. announced CE Mark approval and launch of three new Quartet left ventricular (LV) leads, offering additional electrode spacing options and one new lead shape.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
NeoChord Inc. announced that David H. Adams, M.D., and Michael A. Borger, M.D., Ph.D., will serve as national co-principal investigators of the company’s U.S. pivotal trial scheduled to commence later this year.

A person’s healthcare records contain a wealth of sensitive information. This does not involve just their medical ...
AliveCor, Inc., the leader in FDA-cleared electrocardiogram (ECG) technology for mobile devices, announced today the ...


 March 31, 2016
March 31, 2016