The volume of information attendees receive at the annual Transcatheter Cardiovascular Therapeutics (TCT) interventional ...
October 27, 2016 — BioTrace Medical Inc. said it received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
Philips recently announced its latest image guidance solutions to be featured at the 2016 Transcatheter Cardiovascular Therapeutics meeting (TCT) in Washington, D.C., Oct. 29 – Nov. 2, 2016. Highlights will include the introduction of instant wave-Free Ratio (iFR) co-registration, which integrates iFR pullback data with the angiogram, and the third generation of HeartNavigator, live image guidance software for advanced structural heart disease procedures.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
October 26, 2016 — The U.S. Food and Drug Administration (FDA) provided an update and additional information regarding ...
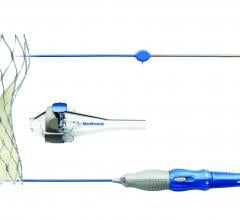
Medtronic announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the CoreValve Evolut R 34 mm valve, now the largest sized transcatheter aortic valve replacement (TAVR) system available in the United States.
Stereotaxis Inc. and Robert Wood Johnson University Hospital (RWJUH) announced that Zyad Younan, M.D., has completed more than 500 cardiac ablation procedures using the Niobe remote magnetic navigation system. Younan currently leads the Northeast region in catheter ablations performed using the Niobe system in the six-and-a-half years since installation.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A recent study from University of Alabama at Birmingham (UAB) researchers published in PLOS ONE compares different available treatments for stroke prevention in patients with non-valvular atrial fibrillation (NVAF).
The Human Animal Bond Research Initiative (HABRI) announced it has awarded a $44,000 grant to Duke University School of Medicine’s Division of Pediatric Cardiology for a new research study on the impact of therapy dogs on pediatric echocardiograms.

October 25, 2016 — St. Jude Medical is recalling 251,346 of its Fortify, Unify and Assura implantable cardioverter ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 24, 2016 — Conventional smart clothing uses conductive fibers or rubber as sensing electrodes, and cardiac ...
(Editor’s note: This is the second part of a two-part series on the proposed Medicare five-year demonstration for a ...
October 24, 2016 — Medtronic received U.S. Food and Drug Administration (FDA) 510(k) clearance for the HawkOne ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
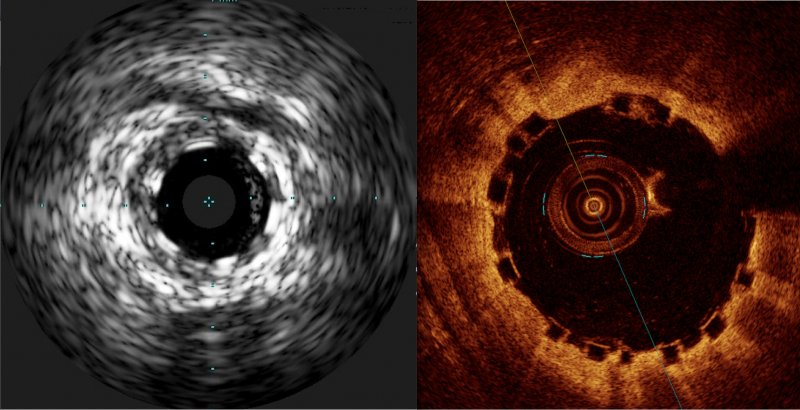
There has been a lot of interest in the interventional community regarding the Abbott Absorb Bioresorbable Vascular ...
October 20, 2016 — SentreHeart Inc. announced in late September the closing of a $35 million Series D round of financing ...
Avinger Inc. recently announced that the company has received expanded indications from the U.S. Food and Drug Administration (FDA) recognizing the Pantheris Lumivascular atherectomy system as a technology that can be used for both therapeutic and diagnostic purposes.

 October 27, 2016
October 27, 2016