CardioKinetix Inc. announced this week that 500 patients have received the company’s Parachute Ventricular Partitioning Device for heart failure. Patients have been treated in more than 15 countries, including patients in key international markets where the device is commercially available, and patients enrolled in PARACHUTE IV, the company’s U.S. pivotal trial under investigational device exemption (IDE).
People who develop heart failure after their first heart attack have a greater risk of developing cancer when compared to first-time heart attack survivors without heart failure, according to a recent study in the Journal of the American College of Cardiology.
Some have labeled bioresorbable scaffolds (BRS), also known as bioresorbable stents, as the fourth evolution of ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 21, 2016 — A new paper published in the June issue of Computer cautions that while mobile health (mHealth) is ...
A novel study has found a simple pre-operative echocardiographic measurement of the amount of torsion of the heart predicted outcomes of mitral valve surgery in some heart failure patients. The study was published in JACC: Basic to Translational Science.
New data from the TITAN II trial confirm the safety and efficacy of the Carillon Mitral Contour System in the treatment of functional (secondary) mitral regurgitation (FMR).
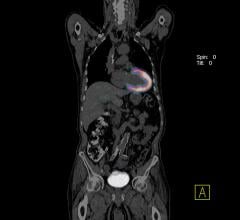
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
At the end of June, experts from three different medical societies released a new guideline to help optimize lifetime management of patients with transposition of the great arteries, a congenital heart defect, both before and after surgical intervention.
July 19, 2016 — Siemens Healthineers recently introduced an expanded Services portfolio, known as Enterprise Services ...
Mitralign Inc. announced last week it has completed subject enrollment in the SCOUT early feasibility study in the United States.
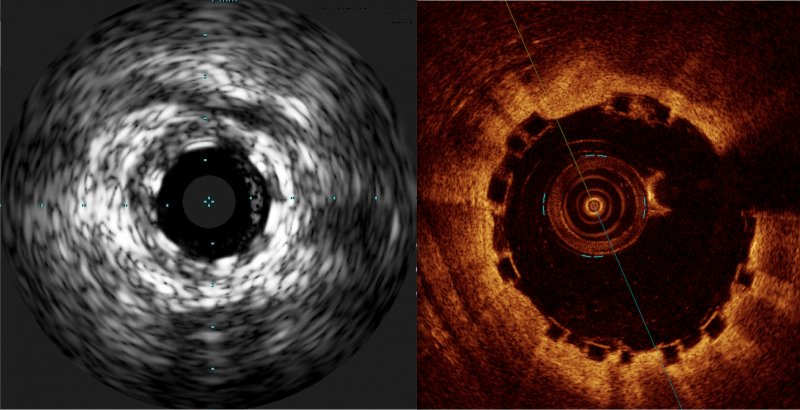
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Dictum Health Inc. announced the launch of a post-U.S. Food and Drug Administration (FDA)-approval study of its end-to-end telehealth care-delivery system with University of California Davis Medical Center in Sacramento.
You have bigger priorities than managing inventory. Patient safety, quality care and clinician satisfaction top the list ...
Good Samaritan Hospital, Los Angeles, is the first hospital on the West Coast to offer patients with coronary artery disease (CAD) Abbott's Absorb dissolving heart stent. Absorb is a drug-eluting coronary stent that dissolves, completely and naturally, in the body over time.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
July 19, 2016 - An updated joint American Society of Nuclear Cardiology (ASNC) imaging guidelines and Society of Nuclear ...
Blood pressure technology company SunTech Medical received U.S. Food and Drug Administration (FDA) approval for distribution of its next generation spot-check vital signs device, the SunTech CT40. Driven by SunTech’s clinical-grade Advantage BP technology, the new spot-check vital signs device provides accurate and reliable automated measurements of blood pressure, temperature and pulse oximetry with clinical-grade performance, advanced features and digital connectivity.
Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for the IN.PACT Admiral drug-coated balloon (DCB) in longer, 150 mm lengths. The new 150 mm-length balloon — available in 4, 5 and 6 mm diameters — will provide greater treatment options for long lesions in patients with peripheral artery disease (PAD).


 July 22, 2016
July 22, 2016