Abbott announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labeling for both the Assurity MRI pacemaker and the Tendril MRI pacing lead. Patients implanted with these low-voltage devices will have the ability to undergo full body magnetic resonance imaging (MRI) scans, if required. With the approval, the Assurity MRI pacemaker is now the world's smallest, longest-lasting wireless MRI-compatible pacemaker, according to Abbott.
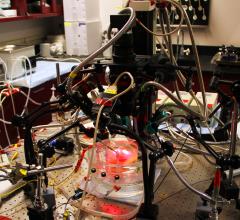
Researchers have developed a technique that allows them to revive parts of human hearts in the laboratory for up to 12 hours while they search for hidden sources of irregular heartbeats.
As the useful applications of big data in the pharmaceutical and healthcare sector become increasingly apparent, 73 percent of organizations in the industry are set to begin or increase investment in big data within the next five years, according to business intelligence provider GBI Research.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
How healthy is your cath lab supply chain? Do you struggle with managing inventory across your cath lab supply chain? It ...
Baylor Heart and Vascular Services at Fort Worth in November became the first program in Texas to implant the Amplatzer PFO Occluder. The device is designed to help reduce the risk of recurrent cryptogenic strokes in patients diagnosed with a patent foramen ovale (PFO) – a small opening between the upper chambers of the heart.
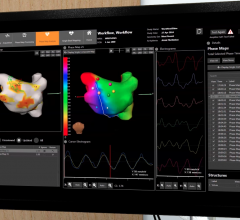
This video, provided by Medtronic, demonstrates the CardioInsight electro-anatomical mapping system. It was cleared by ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 1, 2017 — Medtronic received U.S. Food and Drug Administration (FDA) 510(k) clearance for the CardioInsight ...
A new national survey by Orlando Health found that most women are unaware of the age at which heart screenings should begin. The American Heart Association recommends women begin undergoing regular heart screenings at age 20, but the survey found the majority of women, 60 percent, thought screenings didn't need to begin until after age 30, at least a full decade later.
Heart Test Laboratories Inc. (HTL) has successfully raised $12 million by way of a Common Stock private placement offering. The offering was significantly over-subscribed and provides HTL with a strong financial base entering into 2017. The company expects to commence the international launch of its MyoVista product in the first quarter.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
To save the life of an 11-year-old boy, Ann & Robert H. Lurie Children’s Hospital of Chicago has become the first pediatric hospital in the United States to implant the 50cc SynCardia temporary Total Artificial Heart.
Cigna and HeartWell LLP have launched a program to improve quality and cost of care for people diagnosed with chronic coronary artery disease.
W. L. Gore & Associates (Gore) announced that the Gore Viabahn VBX Balloon Expandable Endoprosthesis (VBX Stent Graft) has received U.S. Food & Drug Administration (FDA) approval for treatment of de novo or restenotic lesions found in iliac arteries. The approval includes lesions at the aortic bifurcation. This marks the availability of the only balloon expandable stent graft with an indication for the iliac artery.
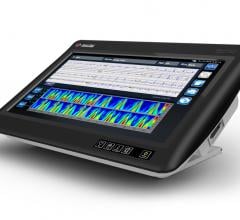
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
January 31, 2017 — The American College of Cardiology (ACC) issued a statement against President Donald Trump’s recent ...
January 31, 2017 — Bard Peripheral Vascular Inc. is recalling the Halo One Thin-Walled Guiding Sheath because the sheath ...
Annual waste for high-value medical devices continues to grow at a rapid pace. With bundled payments putting increased ...


 February 02, 2017
February 02, 2017