Having a non-O blood group is associated with a higher risk of heart attack, according to research presented at Heart Failure 2017 and the 4th World Congress on Acute Heart Failure, April 29-May 2 in Paris, France.

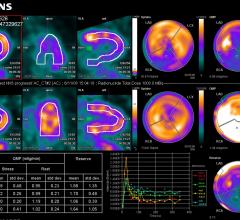
May 30, 2017 — The Society for Cardiovascular Angiography and Interventions (SCAI) 2017 Scientific Sessions took place ...
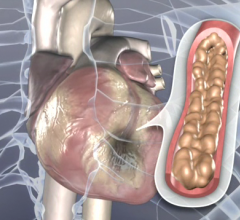
May 1, 2017 — The U.S. Food and Drug Administration (FDA) approved Medtronic’s Resolute Onyx Drug-eluting Stent (DES) ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
This video, provided by Medtronic, demonstrates the Resolute Onyx coronary stent. It was cleared by the FDA and launched ...
Prevencio Inc. announced the publication of data demonstrating that a simple new blood test is more accurate than evaluating commonly-used risk factors in determining whether a person will have a heart attack, stroke or major cardiac event. Researchers believe the data, published in the American Journal of Cardiology (AJC), could revolutionize how doctors identify patients — not only higher-risk patients who need aggressive treatment, but also lower-risk patients who could avoid expensive and invasive procedures.

Tracking cardiovascular device inventory in cath labs, electrophysiology (EP) labs and operating rooms (ORs) can be a ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Researchers at the Australian Nuclear Science and Technology Organisation (ANSTO) have led the development of a new method for producing positron emission tomography (PET) radiotracers. The discovery utilizes the transition metal rhenium to promote fluorine-18 radiolabelling under aqueous, low temperature conditions.
Recent clinical study data presented at the American College of Cardiology (ACC) 2017 meeting show new treatment ...
Biotronik’s Pulsar-18 bare metal stent (BMS) has yielded high primary patency in a real-world setting, according to the 12-month results of the BIOFLEX PEACE all-comers registry. The findings were presented this week at the Charing Cross Symposium, April 25-28 in London. Outcomes of the BIOLUX 4EVER study were also presented on the main stage, revealing 12-month results numerically comparable to drug-eluting stents (DES) when the Pulsar-18 was used in combination with the Passeo-18 Lux drug-coated balloon (DCB).
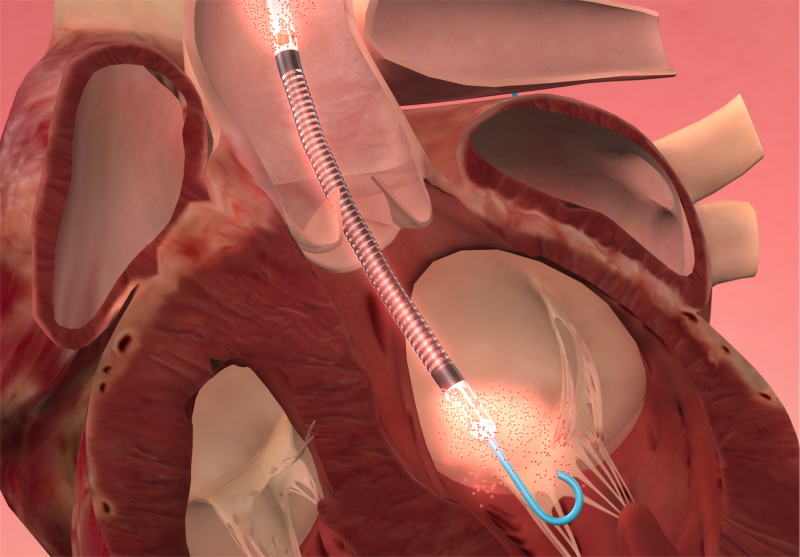
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
David Wolinsky, M.D., director of nuclear cardiology at Cleveland Clinic Florida and past-president of the American ...
Wentworth-Douglass Hospital in Dover, N.H., installed Toshiba Medical’s Infinix-i Sky + angiography system in its endovascular hybrid operating room (OR). Wentworth-Douglass is using the system to diagnose and treat a wide range of vascular disorders, such as aneurysms and peripheral vascular disease.
The vacancy rate for radiographers increased to 4.2 percent in 2017, according to the latest American Society of Radiologic Technologists (ASRT) Radiologic Sciences Staffing and Workplace Survey.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
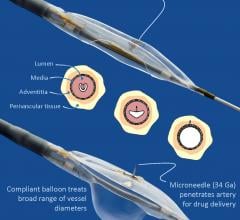
Mercator MedSystems Inc. announced the first patient enrollment into the TANGO (Temsirolimus Adventitial Delivery to Improve Angiographic Outcomes Below the Knee) clinical trial. TANGO will study the effects of using Mercator’s proprietary Bullfrog Micro-Infusion Device for the adventitial delivery of the drug Torisel (temsirolimus) after revascularization of lesions below the knee (BTK) in patients with critical limb ischemia (CLI).
A new Varicose Vein Registry has begun producing useful outcomes information, as reported in the May edition of the Journal of Vascular Surgery: Venous and Lymphatic Disorders. The registry is a joint effort by the Society for Vascular Surgery, the Vascular Quality Initiative and the American Venous Forum. It was launched in January 2015 and was set up to track systematically the outcomes of various treatments for varicose veins, ultimately providing guidance for both consumers and treating physicians.
Boston Scientific announced results from the RANGER SFA trial for the Ranger Paclitaxel-Coated PTA Balloon Catheter at the Charing Cross Symposium, in London. Data demonstrated that the drug-coated balloon (DCB) exhibited both a high rate of primary patency and freedom from target lesion revascularization (TLR) at 12 months, reducing the need for re-interventions to re-establish flow in previously blocked blood vessels.

 May 01, 2017
May 01, 2017