American Regent, a member of the Daiichi Sankyo Group, announced that the first patient has been enrolled into the phase 3 clinical trial, HEART-FID. This double-blind, multicenter, prospective, randomized, placebo-controlled study will assess the efficacy and safety of iron therapy using intravenous (IV) ferric carboxymaltose (FCM), relative to placebo, in the treatment of patients with heart failure, iron deficiency and a reduced ejection fraction.
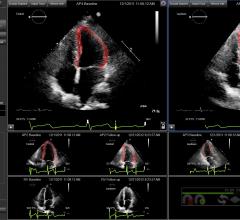
Epsilon Imaging Inc. announced a research study using EchoInsight was presented at the American College of Cardiology (ACC) 2017 Annual Scientific Session and Expo conference from a team at Northwestern University.
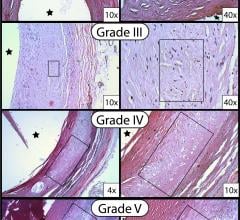
May 3, 2017 — A new study published in The Anatomical Record finds that peripheral arteries, easily accessible by ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The Baylor Jack and Jane Hamilton Heart and Vascular Hospital (BHVH) transcatheter aortic valve replacement (TAVR) team recently placed the first commercially approved CoreValve Evolut Pro system in Texas. This is also the first Evolut Pro valve placed in a failed bioprosthetic (tissue) aortic valve in the United States.
Kim A. Williams, Sr., M.D., chief of cardiology at Rush University Medical Center, Chicago and former president of both ...
Tom Kloetzly, sales and marketing VP for Shimadzu Medical Systems USA, explains the evolution of Shimadzu Corporation since its founding 142 years ago.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
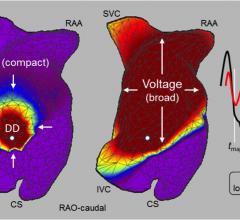
Acutus Medical recently announced that enrollment has been completed in its pivotal Utilizing Novel Dipole Density Capabilities to Objectively Visualize the Etiology of Rhythms in Atrial Fibrillation (UNCOVER AF) study. The study is evaluating the AcQMap High Resolution Imaging and Mapping System in patients with persistent atrial fibrillation (AF) in Europe and Canada.
CardioDx Inc. recently announced the publication of results from the multi-center, community-based PRESET Registry in the peer-reviewed journal, The American Journal of Medicine.
The recently formed group Enhanced Recovery After Cardiac Surgery (ERACS) hosted an organizing session in Boston on April 29 to address the need to standardize best practices in cardiac surgery. The session, titled “ERACS: Best Practices, Cost-Effective Initiative,” featured a consortium of forward-thinking leaders in cardiac surgery joining together as other specialties have done through their Enhanced Recovery After Surgery (ERAS) programs.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
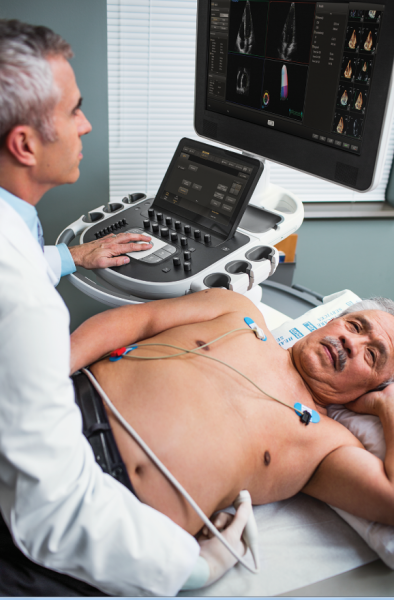
Cardiac ultrasound technology is undergoing an automation technology transformation similar to that seen with computed ...
May 2, 2017 — The U.S. Food and Drug Administration (FDA) granted 510(k) clearance for the Teleflex Inc. AC3 Optimus ...

May 2, 2017 — Here is the list of the top 20 most popular pieces of content on the Diagnostic and Interventional ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
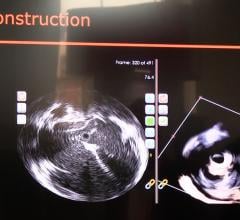
Conavi Medical Inc. announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Foresight ICE (intracardiac echo) System expanded feature set. The enhancements include color doppler, pulsed wave doppler, 2-D and 3-D measurements, and electrocardiogram (ECG)-gated 3-D image acquisition. These enhancements are expected to help electrophysiologists and interventional cardiologists in the United States by providing them with the tools to make decisions during minimally invasive cardiovascular procedures.
ClearFlow Inc. has received the prestigious 2017 Global Frost & Sullivan Award for New Product Innovation. The award was presented to ClearFlow for its development of the PleuraFlow Active Clearance Technology (ACT) System, which has been clinically shown to reduce the occurrence of complications after cardiac surgery, according to the company.
Corindus Vascular Robotics Inc. announced that Fu Wai Hospital in China performed and broadcast live a percutaneous coronary intervention (PCI) procedure using the CorPath GRX System to more than 4,000 attendees at the 22nd annual TCT Asia-Pacific Cardiovascular Summit.

 May 03, 2017
May 03, 2017