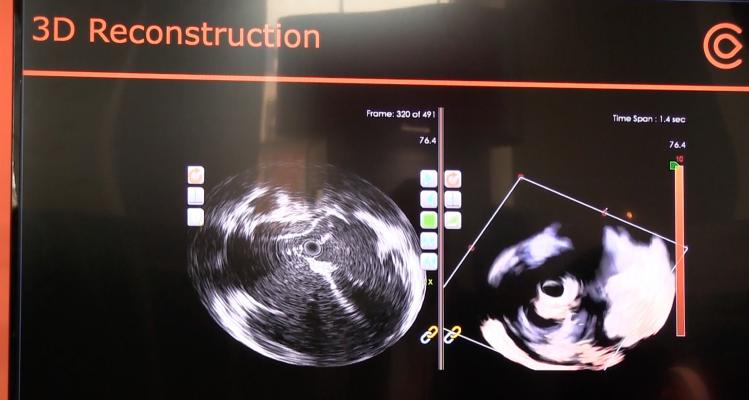
May 1, 2017 — Conavi Medical Inc. announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Foresight ICE (intracardiac echo) System expanded feature set. The enhancements include color doppler, pulsed wave doppler, 2-D and 3-D measurements, and electrocardiogram (ECG)-gated 3-D image acquisition. These enhancements are expected to help electrophysiologists and interventional cardiologists in the United States by providing them with the tools to make decisions during minimally invasive cardiovascular procedures.
The system's indication for use has been expanded to include the ability to assess physiology with its new doppler capabilities. This will expand the range of clinical scenarios in which the Foresight ICE System can be used, according to Conavi Medical CEO Brian Courtney, M.D.
The Foresight ICE System is available for electrophysiologists and interventional cardiologists practicing in the U.S. who are seeking to use advanced technology for their intracardiac procedures.
Conavi will be showcasing its new features at the Heart Rhythm Society 2017 conference, May 10-12 in Chicago.
For more information: www.conavi.com


 October 09, 2025
October 09, 2025