August 28, 2017 — Philips Healthcare and HeartFlow Inc. announced they entered into a collaboration agreement to improve ...
Prem Soman, M.D., director of nuclear cardiology at the Heart and Vascular Institute, University of Pittsburgh, and ...
Randy Thompson, M.D., attending cardiologist, St. Luke’s Mid-America Heart Institute, Kansas City, explains protocols ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Merit Medical Systems Inc. will partner with internationally renowned interventional cardiologist Ferdinand Kiemeneij, M.D., Ph.D., to deliver the next generation in educational opportunities for interventional cardiologists and interventional radiologists.
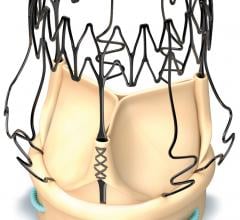
PinnacleHealth is the first hospital in Pennsylvania and one of the first 10 in the country to introduce new technology shown to help protect patients from the risk of stroke during transcatheter aortic valve replacement (TAVR).
Central Minnesota residents now have access to advanced computed tomography (CT) technology that is safe and fast at St. Cloud Hospital with the installation of the Aquilion One/Genesis Edition from Toshiba Medical, a Canon Group company. The system is located across a hallway from the emergency department and is being used for a wide range of exams, including neuro perfusion studies.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Medical Device Innovation, Safety and Security Consortium (MDISS) recently launched the first of more than a dozen planned device security testing labs and cyber-ranges. The new MDISS World Health Information Security Testing Lab (WHISTL) facilities will comprise a federated network of medical device security testing labs, independently owned and operated by MDISS-member organizations including healthcare delivery organizations, medical device manufacturers, universities and technology companies. Each WHISTL facility will launch and operate under a shared set of standard operating procedures. The goal is to help organizations work together to more effectively address the public health challenges arising from cybersecurity issues emergent in complex, multi-vendor networks of medical devices.
August 22, 2017 — LivaNova PLC announced its Perceval sutureless aortic heart valve received approval from the Centers ...
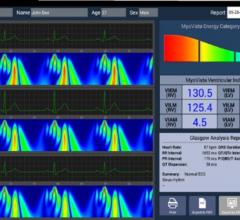
HeartSciences announced the European launch of the MyoVista high sensitivity electrocardiograph (hsECG) Testing Device, developed in response to the global unmet need for effective, low-cost, front-line screening of cardiac disease in both symptomatic and asymptomatic patients. MyoVista measures the heart's energy during each heartbeat using a type of advanced signal processing known as Continuous Wavelet Transform (CWT).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
The U.S. Department of Health and Human Services (HHS), Office for Civil Rights (OCR) recently launched a revised web tool that puts important information on cybersecurity breaches into the hands of individuals. The tool is designed to empower them to better identify recent breaches of health information and to learn how all breaches of health information are investigated and successfully resolved.
Biotronik announced U.S. Food and Drug Administration (FDA) approval and commercial availability of Edora HF-T QP, an MR conditional quadripolar (QP) cardiac resynchronization therapy pacemaker (CRT-P) with MRI (magnetic resonance imaging) AutoDetect technology.
Medtronic plc announced a global randomized clinical trial that will evaluate one-month dual antiplatelet therapy (DAPT) in patients implanted with the Resolute Onyx Drug-Eluting Stent (DES) during percutaneous coronary intervention (PCI). Designed to evaluate clinical DAPT outcomes between two DES for the first time ever, the RESOLUTE ONYX ONE-MONTH DAPT Study intends to help inform DAPT guidelines for newer-generation DES that currently favor bare-metal stents (BMS) for patients with stable ischemic heart disease who might require a shorter dual antiplatelet regimen.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
August 18, 2017 — The American Society of Nuclear Cardiology (ASNC) has released a joint expert consensus document with ...

August 17, 2017 — Cybersecurity has become a growing concern in healthcare as patient data, medical systems and ...
Houston Methodist Hospital and Siemens Healthineers have entered into a multi-year agreement to bring cutting-edge technology to Houston Methodist that is not currently available in the Texas Medical Center. This agreement will provide innovative, world-class medical technology to Houston Methodist Hospital and all of its community hospital facilities.


 August 28, 2017
August 28, 2017