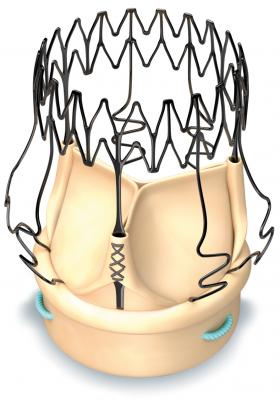
August 22, 2017 — LivaNova PLC announced its Perceval sutureless aortic heart valve received approval from the Centers for Medicare and Medicaid Services (CMS) for a New Technology Add-on Payment (NTAP). The Perceval valve met the CMS criteria for NTAP, including the demonstration of substantial clinical improvement over existing technologies. Beginning on Oct. 1, 2017, CMS has stated it will reimburse hospitals for the Perceval valve procedure with the Medicare Severity Diagnosis Related Group (MS-DRG) payment they normally receive, plus an additional payment of up to $6,110.23.
Clinical trial data has demonstrated the Perceval valve’s ability to optimize the overall surgical approach for cardiac surgeons through reduced procedure times, decreased postoperative complications and shorter hospital stays. The Perceval valve is suitable for traditional surgery and also enables minimally invasive surgical approaches through its collapsible design and sutureless deployment. Engineered to restore natural valve performance, the Perceval valve features a super-elastic stent, which is able to adapt to the movement of the aorta during the cardiac cycle and provide excellent hemodynamics.
Perceval received U.S. Food and Drug Administration (FDA) approval in 2016, but has been in clinical use worldwide for 10 years and studied in more than 190 publications. Severe aortic stenosis affects over 500,000 Americans and 85,000 patients undergo aortic valve replacement each year.
Read the article "LivaNova Announces Positive Data for Perceval Sutureless Valve at AATS 2017."
For more information: www.livanova.com


 February 06, 2026
February 06, 2026 









