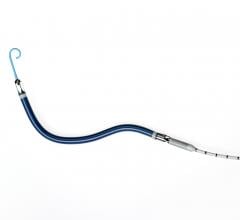
Avinger Inc. recently announced Conformité Européenne (CE) Marking approval for treating in-stent restenosis with the Pantheris Lumivascular atherectomy system.
September 28, 2017 — New research revealed that on average, more than 75 percent of people aged 65 and older worldwide ...
Farouc Jaffer, M.D., Ph.D., director of coronary interventions at Massachusetts General Hospital, discusses the newest ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 28, 2017 — The AstraZeneca HealthCare Foundation’s Connections for Cardiovascular Health (CCH) program is ...
Abiomed Inc. recently received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) for the Impella RP heart pump. Culminating from five years of research, this approval follows the prior FDA Humanitarian Device Exemption (HDE) received in January 2015 and adds the Impella RP heart pump to Abiomed's platform of PMA-approved devices.
September 27, 2017 — Boston Scientific recently launched the Resonate family of implantable cardioverter defibrillator ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Toshiba Medical announced it will highlight several of its latest vascular and interventional imaging solutions at the 2017 Annual Meeting of the Radiological Society of North America (RSNA), Nov. 26-Dec. 1 in Chicago.
Spectranetics is recalling its Bridge Occlusion Balloon Catheter due to the possibility of a blocked guidewire lumen in some device units. If a device with a blocked guidewire lumen were to be used during the procedure, the device would not be positioned correctly and hemorrhage would not be controlled. This would delay life-saving treatment, which may result in immediate and serious adverse health consequences, including death.
BioCardia Inc. recently announced 12-month results from the Phase II TRIDENT clinical trial, conducted by the University of Miami Miller School of Medicine and co-sponsored by the company. The results showed a positive safety profile for allogeneic, or donor cell-based, mesenchymal stem cells delivered with the company’s Helix transendocardial delivery system at 30 days. The study was concurrently published in Circulation Research and presented on the podium at the Heart Failure Society of America (HFSA) Annual Scientific Meeting, Sept. 16-19 in Dallas. Results were presented by Victoria Florea, M.D., of the Interdisciplinary Stem Cell Institute at the University of Miami.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
September 26, 2017 — The one-year results of the SENTRY clinical trial were presented by principal investigator Michael ...
Treatment of heart attack patients depends on their history of cancer, according to research published recently in European Heart Journal: Acute Cardiovascular Care. The study in more than 35,000 heart attack patients found they were less likely to receive recommended drugs and interventions, and more likely to die in hospital if they had cancer than if they did not.
BioCardia Inc. announced the trial design for its pivotal Phase III CardiAMP Heart Failure Trial was presented during the “Heart Failure: The Big Target for CV Regenerative Therapy State of the Field” session at the Texas Heart Institute International Symposium on Cardiovascular Regenerative Medicine, Sept. 15-16 in Houston. As an invited faculty member for the meeting, Professor of Cardiovascular Medicine at the University of Florida and study co-national principal investigator Carl Pepine, M.D., presented the design.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Physicians demonstrated that reducing metal burden in superficial femoral artery (SFA) therapy could effectively reduce restenosis rates, according to results from various Biotronik studies. The results were presented at the 2017 congress of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE), Sept. 16-20 in Copenhagen, Denmark.
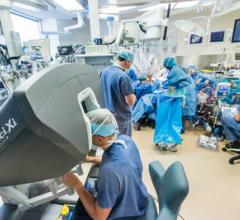
The Montreal Heart Institute (MHI) announced the acquisition of the da Vinci Xi, a new-generation surgical robot, and the first in Canada to be exclusively dedicated to cardiac surgery. "The development of robotic cardiac surgery plays a major role in the expansion of minimally invasive surgery, whose purpose is to minimize the trauma endured by the body by reducing the incision size, for instance", according to the surgical team counting among its members Michel Pellerin, M.D., and Denis Bouchard, M.D., the two cardiac surgeons who performed the first robotic mitral intervention last April. "This new technology will improve the patients' quality of life and allow for a faster return to daily activity, among other benefits", they continued.
Abbott announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labeling for one of the company's most widely-used implantable cardioverter defibrillators (ICD) and associated high voltage leads. The approval of MR-conditional labeling for the Ellipse ICD with the Tendril MRI pacing lead and Durata and Optisure high voltage leads adds another patient-centric benefit to the device and will help further improve access for patients suffering from abnormally fast heart rhythms who need an ICD and who may need an MRI scan in the future.


 September 29, 2017
September 29, 2017