
July 26, 2018 — A new multi-societal expert consensus document has been released that summarizes the position of these ...
July 25, 2018 — The U.S. Food and Drug Administration (FDA) cleared Siemens Healthineers high-sensitivity troponin I ...
Robert C. Hendel, M.D., FACC, FAHA, MASNC, director, Tulane Heart and Vascular Institute, explains the impact the ASNC ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A discussion with Patricia Dickson, LRT(CT), director of imaging and outpatient services, Capital Cardiology Associates ...
July 24, 2018 — In celebration of its 25th anniversary, the Journal of Nuclear Cardiology (JNC) is highlighting the top ...
July 23, 2018 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the PocketECG Cardiac ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 24, 2018 - Diagnostic and consumer healthcare remote monitoring technology company Biotricity Inc. has completed ...
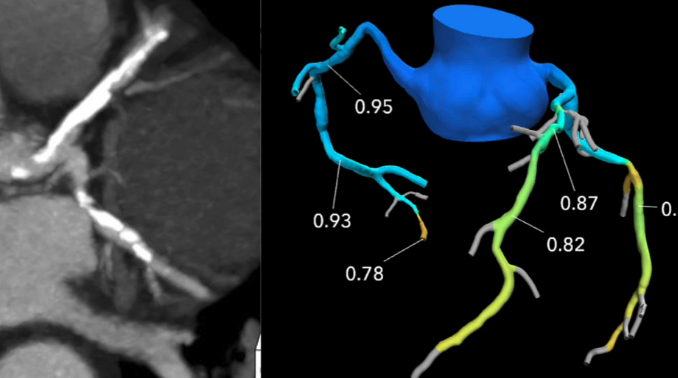
The use of non-invasive fractional flow reserve CT (FFR-CT) was the hottest topic discussed at the Society of ...
Ed Nicol, M.D., FSCCT, MBA, head of cardiac CT, Royal Brompton Hospital, London, and chair of the Society of ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
July 20, 2018 — Boston Scientific Corp. today has signed an agreement to acquire Claret Medical Inc., which has ...
Kavitha Chinnaiyan, M.D., FACC, FSCCT, associate professor, Oakland University, William Beaumont School of Medicine ...
Here are the stories and other content in the July-August 2018 issue of Diagnostic and Interventional Cardiology (DAIC) ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Avinger Inc. announced in June that several physicians have successfully treated over 40 patients for peripheral artery disease (PAD) in the United States across 13 sites with the next-generation Pantheris image-guided atherectomy system. This positive initial case experience in the U.S. adds to the impressive clinical results achieved in the first 30 cases completed in Germany earlier this year and provides a strong foundation for expanded market launch into all U.S. accounts, according to the company.
July 18, 2018 — Abbott announced it received approval from the U.S. Food and Drug Administration (FDA) for a next ...
Suhny Abbara, M.D., FSCCT, chief of cardiothoracic imaging and chair of the CT operations committee, University of Texas ...

 July 26, 2018
July 26, 2018















