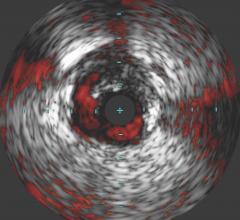
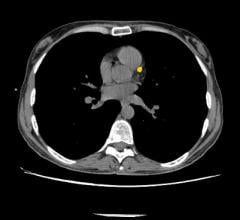
Avinger Inc. announced in June that several physicians have successfully treated over 40 patients for peripheral artery disease (PAD) in the United States across 13 sites with the next-generation Pantheris image-guided atherectomy system. This positive initial case experience in the U.S. adds to the impressive clinical results achieved in the first 30 cases completed in Germany earlier this year and provides a strong foundation for expanded market launch into all U.S. accounts, according to the company.
July 18, 2018 — Abbott announced it received approval from the U.S. Food and Drug Administration (FDA) for a next ...
Suhny Abbara, M.D., FSCCT, chief of cardiothoracic imaging and chair of the CT operations committee, University of Texas ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A discussion with Gianluca Pontone, M.D., Ph.D., FSCCT, director of cardiovascular MRI, Centro Cardiologico Manzino ...
Jonathon Leipsic, M.D., FSCCT, professor of radiology and cardiology at the University of British Columbia, Vancouver ...
An interview with Patrick Serruys, M.D., Ph.D., Imperial College London, principal investigator of the SYNTAX III Trial ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A discussion with Todd Villines, M.D., FACC, FAHA, FSCCT, director of cardiovascular research and cardiac CT programs at ...
A new way to examine stress and inflammation in the heart will help Parkinson’s researchers test new therapies and explore an unappreciated way the disease puts people at risk of falls and hospitalization.
Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for a less-invasive implant approach of its HVAD System, a left ventricular assist device (LVAD) for patients with advanced heart failure. The HVAD System is the smallest commercially available LVAD according to Medtronic, and the only LVAD approved in the U.S. for implant via thoracotomy, a small lateral, surgical incision between the patient's ribs on the left side of the chest.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Endologix Inc. announced one-year results from the LUCY (Evaluation of FemaLes who are Underrepresented Candidates for Abdominal Aortic AneurYsm Repair) registry at the 2018 Society for Vascular Surgery (SVS) Annual Meeting, June 21-23 in Boston. The LUCY study is the first to prospectively evaluate endovascular aneurysm repair (EVAR) outcomes in women who have more complex aortic anatomy and, subsequently, have worse reported outcomes than men undergoing EVAR. The results of the LUCY one-year data expand on the 30-day results presented last year, showing that at least 28 percent more women are eligible for minimally-invasive EVAR when using the Ovation Abdominal Stent Graft System than when using other EVAR systems.
New research published in Experimental Physiology has indicated potential differences in heart health benefits of exercise intensity in teenagers. Teenage years are an important stage of life, with research suggesting it is a time during which heart diseases start to develop. These findings indicate that teenagers who participate in high intensity exercise have lower blood pressure. This may lead to a lower risk of developing heart disease later in life, but this requires confirmation with further research.
Boston Scientific Corp. recently announced a definitive agreement to acquire Cryterion Medical Inc., a privately-held company developing a single-shot cryoablation platform for the treatment of atrial fibrillation (AF). The addition of this cryoballoon platform positions the company as the first to have both cryothermal and radiofrequency (RF) single-shot, balloon-based ablation therapies in its portfolio.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
July 13, 2018 – iSchemaView has signed Diagnostic Imaging Australia (DIA) to be the exclusive distributor for the RAPID ...
Intact Vascular Inc. announced the publication of the iDissection Classification trial results in the current issue of Journal of Invasive Cardiology.
Zebra Medical Vision has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Coronary Calcium Scoring algorithm. The algorithm, capable of automatically calculating a patient’s Agatston equivalent coronary calcium score from an electrocardiogram (ECG)-gated computed tomography (CT) scan, provides physicians with important data used in the assessment of the risk for coronary artery disease.


 July 18, 2018
July 18, 2018