Blood pressure and cholesterol-lowering drugs continue to improve survival in patients with hypertension after more than a decade, according to late breaking results from the ASCOT Legacy study. The results were presented at the European Society of Cardiology (ESC) Congress 2018, Aug. 25-29 in Munich, Germany, and published in The Lancet.
Acarix AB’s ultra-sensitive acoustic CADScor System for coronary artery disease risk assessment will be on display at the European Society of Cardiology (ESC) Congress 2018, Aug. 25-28 in Munich, Germany. The CADScor System has been validated clinically to rule out CAD with 96-97 percent negative predictive value. The device is gaining interest and increased use across Germany and Denmark, key Swedish hospitals are testing the system and expansion in other European countries is under way. At ESC 2018, both in the exhibition booth and at a special session Aug. 28, there will be opportunities to get first-hand information about the CADScor System and to meet experts with experience from using the system in clinical practice.
U.S. Expeditions and Explorations (USX) and Cardiac Insight Inc. announced the successful completion of an expedition on North America’s highest mountain, Denali, to gather data on sudden cardiac death (SCD) risk at high altitudes using the Cardea Solo device.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The National Institutes of Health announced in June it plans to end funding to the Moderate Alcohol and Cardiovascular Health (MACH) trial. The decision is based on concerns about the study design that cast doubt on its ultimate credibility. This includes whether the study would effectively address other significant consequences of moderate alcohol intake, such as cancer.
Cerner recently collaborated with Duke Clinical Research Institute (DCRI) to develop an atherosclerotic cardiovascular disease (ASCVD) Risk Calculator app. The app was designed as a tool to increase communication between the patient and their doctor about ways to live a healthier life and risk factors for heart disease and stroke.
W. L. Gore & Associates Inc. (Gore) announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the Gore Molding & Occlusion Balloon. The compliant polyurethane balloon catheter is designed to assist in the expansion of self-expanding stent grafts or to temporarily occlude large-diameter vessels. The device also received approval from the Japanese Ministry of Health, Labour and Welfare, and receipt of CE Mark. It meets all endovascular aortic repair (EVAR) procedural requirements, according to Gore – a single balloon that replaces the need for multiple molding and occlusion balloons.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 22, 2018 — Philips recently announced the introduction of the Epiq CVx cardiovascular ultrasound system. Built on ...
Breastfeeding is not only good for babies, according to new research in the Journal of the American Heart Association. The research indicates there is growing evidence breastfeeding may also reduce the risk for stroke in post-menopausal women who reported breastfeeding at least one child.
Kim A. Williams, Sr., M.D., MACC, MASNC, FAHA, FESC, cardiology division chief and James B. Herrick professor at Rush ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Rami Doukky, M.D., professor of medicine, preventive medicine and radiology, and chief of the Division of Cardiology at ...
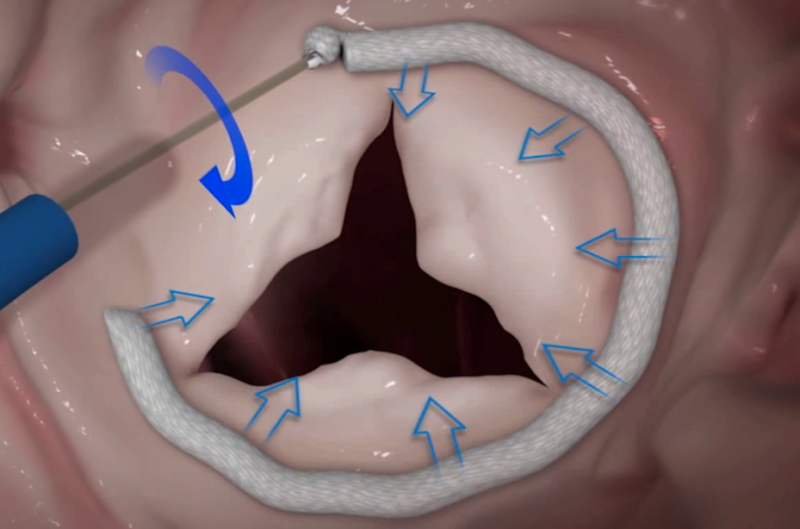
The tricuspid valve has been dubbed “the forgotten valve” by many practitioners in the interventional cardiology space ...
More than 100,000 people have been trained in the life-saving skill of Hands-Only CPR since the American Heart Association launched its Hands-Only CPR training kiosk program in 2016, according to the association. As part of the program that is nationally supported by Anthem Foundation, the AHA has placed 30 of these interactive devices in cities across the country.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Lexington Biosciences Inc. recently announced the completion of the initial HeartSentry study conducted at San Francisco Bay-area Diablo Clinical Research.
iBeat recently announced the release and shipping of its iBeat Heart Watch. The heart and blood flow monitoring smartwatch will engage the user and can notify first responders for immediate medical aid if something appears to be wrong.
NuCryo Vascular announced that the company has signed a commercialization agreement with Lokai Medical, a specialty distributor of coronary/peripheral and interventional devices, to distribute the PolarCath Balloon Dilatation System in the United States.


 August 27, 2018
August 27, 2018
















