Quantum Genomics announced the enrollment of the first patient in its QUORUM Phase IIb study of its lead clinical candidate, firibastat, in patients with heart failure after acute myocardial infarction (AMI). Firibastat is a first-in-class brain aminopeptidase A inhibitor, being developed for the treatment of resistant hypertension and heart failure.
June 14, 2019 – A late-breaking study examined the effects of intravascular ultrasound (IVUS) guided drug-eluting stent ...
June 14, 2019 – An expert consensus statement provides recommendations for optimizing the financial operations of the ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Bardy Diagnostics Inc. announced that its Carnation Ambulatory Monitor (CAM) was recognized with the “Best New Diagnostic Technology” award in the 2019 MedTech Breakthrough Awards Program. MedTech Breakthrough is an independent organization that recognizes the top companies and solutions in the global health and medical technology market. BardyDx earned the distinction for its P-wave centric ambulatory cardiac patch monitoring and arrhythmia detection technology.
Silk Road Medical Inc. announced the presentation of real-world data for the treatment of patients with carotid artery disease at risk for stroke at the Society for Vascular Surgery 2019 Vascular Annual Meeting (VAM), June 12-15 in National Harbor, Md. In a headline presentation, Mahmoud Malas, M.D., of the University of California, San Diego School of Medicine shared updated results for the ongoing TransCarotid Artery Revascularization (TCAR) Surveillance Project.
Three-dimensional (3-D) printing software and solutions company Materialise has received U.S. Food and Drug Administration (FDA) clearance for its Mimics Enlight cardiovascular planning software suite. The first release will support clinicians planning complex transcatheter mitral valve replacement (TMVR) procedures.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Orchestra BioMed Inc. announced it has formed a global strategic partnership with Terumo Corp. for development and commercialization of the Virtue Sirolimus-Eluting Balloon (SEB), one of Orchestra’s lead assets, in the percutaneous coronary and peripheral interventions field.

June 12, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Boston Scientific Corp. has initiated the OPTION trial to compare safety and effectiveness of the next-generation Watchman FLX left atrial appendage closure (LAAC) platform to first-line oral anticoagulants (OAC) for stroke risk reduction in patients with non-valvular atrial fibrillation (AF) who undergo a cardiac ablation procedure. OACs used in the trial will include direct oral anticoagulants (DOAC) and warfarin.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Specialized risk scores derived from testing that calculates the cumulative effect of an individual’s entire DNA sequence may reliably predict heart disease in people who have not yet had a heart attack, according to new research. The research is published in Circulation: Genomic and Precision Medicine, an American Heart Association journal.
In a letter sent to healthcare providers, the U.S. Food and Drug Administration (FDA) validates that Abiomed’s Impella RP heart pump is safe and effective for treatment of right heart failure. The letter comes after the FDA examined the results from Abiomed’s 18-month post-approval study (PAS) of 42 Impella RP patients. The data shows a 64 percent survival rate and 90 percent heart recovery for the subgroup of PAS patients who met the enrollment criteria of Impella RP’s premarket clinical studies. That survival rate is, as the FDA writes in its letter, “similar to the premarket clinical study survival rate,” which was 73 percent.
Centers for Medicare and Medicaid Services (CMS) Administrator Seema Verma addressed the American Medical Association (AMA) Annual Meeting of the House of Delegates, which ran June 8-12 in Chicago. In her remarks, Verma addressed current efforts by the Trump Administration to enact healthcare reform. The following is a transcript of her remarks.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
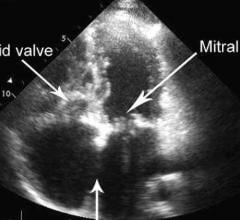
June 11, 2019 — Abbott recently announced positive late-breaking data from its TRILUMINATE study of the company's ...
W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket approval (PMA) of the Gore Cardioform ASD Occluder for the percutaneous closure of ostium secundum atrial septal defects (ASDs). The FDA approval was supported by data collected from the pivotal stage of the Gore ASSURED Clinical Study, which demonstrated 100 percent closure success at the six-month evaluation in patients with a successful implant.
Medivis announced that its augmented reality (AR) technology platform for surgical applications, SurgicalAR, has received 510(k) clearance for clinical use in the operating room by the U.S. Food and Drug Administration (FDA). The New York City-based medical technology company will commence the immediate commercialization of the platform in the United States.


 June 14, 2019
June 14, 2019