The anti-proliferative drug paclitaxel has been used as a coating on coronary stents to prevent restenosis since 2003 ...
January 25, 2019 — Siemens Healthineers presented its first intelligent software assistant for radiology, the AI-Rad ...
January 25, 2019 — Profusa announced promising clinical data from two studies evaluating the company's Lumee Oxygen ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Joe Cleveland, M.D., professor of cardiothoracic surgery, at the University of Colorado Hospital, offers a cardiac ...
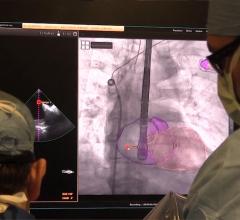
A new report in the Journal of Vascular Surgery chronicles a multi-site randomized controlled trial comparing treatment efficacy, functional outcomes, cost effectiveness and quality of life for various treatments for critical limb ischemia (CLI). The trial will enroll 2,100 patients suffering from CLI, a debilitating and increasingly common manifestation of peripheral arterial disease (PAD) that puts patients at high risk for leg amputation, cardiovascular complications and death.
Researchers have identified another reason to limit red meat consumption: high levels of a gut-generated chemical called trimethylamine N-oxide (TMAO) that is also linked to heart disease. Scientists found that people who eat a diet rich in red meat have triple the TMAO levels of those who eat a diet rich in either white meat or mostly plant-based proteins, but discontinuation of red meat eventually lowers those TMAO levels.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Catheter-based blood clot removal a decade ago was a standard of care for acute coronary revascularization, but declined ...
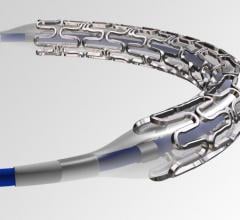
The rise cardiovascular disease has been instrumental in fueling the coronary stent market share in the past few years ...
January 21, 2019 – Medical experts have released “Guidelines for Performing a Comprehensive Transthoracic ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Meat-free athletes have already proven the performance-boosting power of a plant-based diet. Now, “Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports,” a new scientific review published in the journal Nutrients1 adds further evidence that plant-based athletes benefit from improvements in heart health, performance and recovery.

Interventional cardiac resynchronization therapy (I-CRT) describes the repurposing of a set of tools and techniques ...
Abbott announced U.S. Food and Drug Administration (FDA) approval of the TactiCath Contact Force Ablation Catheter, Sensor Enabled, a new ablation catheter designed to help physicians accurately and effectively treat atrial fibrillation (AFib). The approval further expands Abbott's portfolio of cardiac ablation tools that integrate with the company's EnSite Precision cardiac mapping system to help physicians develop more precise images of the heart during cardiac ablation procedures.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Philips announced the launch of Azurion with FlexArm, designed to enhance positioning flexibility for image-guided procedures.

Many of the latest advances in cardiovascular imaging technologies are unveiled each year at the Radiological Society of ...
The U.S. Food and Drug Administration (FDA) issued a letter Jan. 17, 2019, to healthcare providers regarding a recent publication that suggests a possible increased risk of death at two years and beyond in patients treated for a type of peripheral artery disease (PAD) with paclitaxel-coated balloons or paclitaxel-eluting stents. The letter was issued in response to a recent publication in the Journal of the American Heart Association identifying the risk. It recommends doctors continue to monitor these patients, and discuss the benefits and risks of all available treatment options for patients with PAD.

 January 25, 2019
January 25, 2019