March 22, 2019 — The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers ...
Raza Alvi, M.D., a research fellow in radiology at Massachusetts General Hospital, has been involved in a study of a ...

Reflecting a trend toward the increased use of computed tomography (CT) in cardiology, Siemens Healthineers launched a ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Khaldoun Tarakji, M.D., MPH, associate section head, section of electrophysiology and pacing in the Robert and Suzanne ...
Christine Albert, M.D., MPH, director of the Center for Arrhythmia Prevention at Brigham and Women’s Hospital, and ...
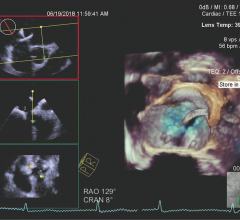
Clinicians should use echocardiography when determining whether patients with heart failure and a leaking heart valve are likely to benefit from valve repair, according to research presented at the American College of Cardiology’s 68th Annual Scientific Session, March 16-18 in New Orleans.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The U.S. Food and Drug Administration (FDA) approved Impulse Dynamics’ Optimizer Smart system for treating patients with chronic, moderate-to-severe heart failure to restore a normal timing pattern of the heartbeat. The device is indicated for patients who are not suited for treatment with other heart failure devices such as cardiac resynchronization therapy. The FDA gave the Optimizer Smart system a Breakthrough Device designation because it treats a life-threatening disease, heart failure, and addresses an unmet medical need in patients who fail to get adequate benefits from standard treatments and have no alternative treatment options.
Results from the landmark Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT) demonstrated Medtronic’s Tyrx Absorbable Antibacterial Envelope reduced the risk of major infection by 40 percent, and pocket infection by 61 percent, in patients with cardiac implantable electronic devices (CIEDs). The improvements were in comparison to standard-of-care pre-operative antibiotics. The trial results were presented in a late-breaking session at the American College of Cardiology’s 68th Annual Scientific Sessions (ACC.19), March 16-18 in New Orleans, and published simultaneously in The New England Journal of Medicine.
At the American College of Cardiology’s (ACC) annual meeting, March 16-18 in New Orleans, Philips announced the results of the DEFINE PCI [1] study, which assessed the level of residual ischemia found in patients after percutaneous coronary interventions (PCI). This study found that 1 in 4 patients [1] treated with standard-of-care PCI left the cath lab with residual ischemia (iFR < 0.90), as demonstrated by using a blinded instantaneous wave-free ratio (iFR) pullback measurement, which is Philips’ new physiologic guidance technology.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
March 20, 2019 — Cook Medical is recalling one lot of its Transseptal Needle due to a manufacturing error that resulted ...
Prevencio Inc. announced data confirming the high accuracy of its artificial intelligence (AI)-driven, multiple-protein HART CVE Test for predicting cardiovascular events (CVE) and HART CAD Test for diagnosing coronary artery disease (CAD). Researchers believe these findings, presented at the 2019 American College of Cardiology (ACC) Scientific Sessions, March 16-18 in New Orleans, demonstrate the robustness and accuracy of these tests. The new data, from two additional hospitals, confirm results previously published from Massachusetts General Hospital and James Januzzi, M.D.
iSchemaView announced the company’s co-founder Gregory Albers, M.D., has received the Distinguished Clinical Research Achievement Award, presented by the Clinical Research Forum. The Distinguished Clinical Research Achievement Awards are presented to the top two studies that show creativity, innovation, or a novel approach that demonstrates an immediate impact on the health and well-being of patients.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
The Bristol-Myers Squibb-Pfizer Alliance announced results from the Phase 4 AUGUSTUS trial evaluating Eliquis (apixaban) versus vitamin K antagonists (VKAs) in patients with non-valvular atrial fibrillation (NVAF) and recent acute coronary syndrome (ACS) and/or undergoing percutaneous coronary intervention (PCI). Results show that in patients receiving a P2Y12 inhibitor with or without aspirin (antiplatelet therapies), the proportion of patients with major or clinically relevant non-major (CRNM) bleeding at six months was significantly lower for those treated with Eliquis compared to those treated with a VKA (10.5 percent vs. 14.7 percent, respectively; hazard ratio [HR]: 0.69, 95 percent confidence interval [CI]: 0.58-0.81; p-superiority<0.001).
Late-breaking results confirm the HeartFlow FFRct (fractional flow reserve computed tomography) Analysis enables efficient identification of which patients, despite symptoms suggestive of coronary artery disease (CAD), have a low risk of adverse cardiovascular events and can safely avoid invasive testing out to one year. These results from the ADVANCE trial were presented as a late-breaking trial during the American College of Cardiology’s (ACC) 68th Annual Scientific Session, March 16-18 in New Orleans, and simultaneously published in the Journal of the American College of Cardiology (JACC): Cardiovascular Imaging.
The American College of Cardiology (ACC) released a list of the latest practice-changing presentations at the ACC.19 ...

 March 22, 2019
March 22, 2019