Phase 3 results from Study 2, also known as CLEAR WISDOM, of bempedoic acid were recently presented at the American College of Cardiology (ACC) Scientific Sessions & Expo, March 16-18 in New Orleans. Bempedoic acid is being developed as a complementary, cost-effective, convenient, once-daily, oral therapy for the treatment of patients with elevated low-density lipoprotein cholesterol (LDL-C). Bempedoic acid and the bempedoic acid/ezetimibe combination tablet new drug applications have been submitted to the U.S. Food and Drug Administration (FDA), and are under regulatory review for marketing authorization by the European Medicines Agency.
Severely ill patients with advanced heart failure who received the HeartMate 3 left ventricular assist device (LVAD) suffered significantly fewer strokes, pump-related blood clots and bleeding episodes after two years, compared with patients who received an older pump. The data came from research presented at the American College of Cardiology’s 68th Annual Scientific Session, March 16-18 in New Orleans.
Magid Awadalla, MBBS, is an advanced cardiac imaging research fellow at Massachusetts General Hospital. He has been ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Among patients with severe symptomatic aortic stenosis at low surgical risk, transcatheter aortic valve replacement (TAVR) using the Sapien 3 valve compared with conventional surgery significantly reduced the primary endpoint of death, stroke and re-hospitalizations by 46 percent at one year. The data, from the latest PARTNER trial, were presented at the American College of Cardiology’s 68th Annual Scientific Session, March 16-18 in New Orleans. In addition, the rates of death from any cause, stroke and repeat hospitalizations independently favored TAVR at 30 days and at one year, researchers said.
Troponins are a family of proteins found in skeletal and heart (cardiac) muscle fibers that produce muscular contraction ...

March 22, 2019 — The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Raza Alvi, M.D., a research fellow in radiology at Massachusetts General Hospital, has been involved in a study of a ...

Reflecting a trend toward the increased use of computed tomography (CT) in cardiology, Siemens Healthineers launched a ...
Khaldoun Tarakji, M.D., MPH, associate section head, section of electrophysiology and pacing in the Robert and Suzanne ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Christine Albert, M.D., MPH, director of the Center for Arrhythmia Prevention at Brigham and Women’s Hospital, and ...
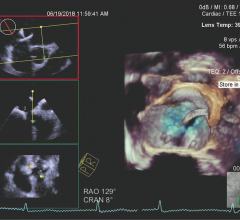
Clinicians should use echocardiography when determining whether patients with heart failure and a leaking heart valve are likely to benefit from valve repair, according to research presented at the American College of Cardiology’s 68th Annual Scientific Session, March 16-18 in New Orleans.
The U.S. Food and Drug Administration (FDA) approved Impulse Dynamics’ Optimizer Smart system for treating patients with chronic, moderate-to-severe heart failure to restore a normal timing pattern of the heartbeat. The device is indicated for patients who are not suited for treatment with other heart failure devices such as cardiac resynchronization therapy. The FDA gave the Optimizer Smart system a Breakthrough Device designation because it treats a life-threatening disease, heart failure, and addresses an unmet medical need in patients who fail to get adequate benefits from standard treatments and have no alternative treatment options.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Results from the landmark Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT) demonstrated Medtronic’s Tyrx Absorbable Antibacterial Envelope reduced the risk of major infection by 40 percent, and pocket infection by 61 percent, in patients with cardiac implantable electronic devices (CIEDs). The improvements were in comparison to standard-of-care pre-operative antibiotics. The trial results were presented in a late-breaking session at the American College of Cardiology’s 68th Annual Scientific Sessions (ACC.19), March 16-18 in New Orleans, and published simultaneously in The New England Journal of Medicine.
At the American College of Cardiology’s (ACC) annual meeting, March 16-18 in New Orleans, Philips announced the results of the DEFINE PCI [1] study, which assessed the level of residual ischemia found in patients after percutaneous coronary interventions (PCI). This study found that 1 in 4 patients [1] treated with standard-of-care PCI left the cath lab with residual ischemia (iFR < 0.90), as demonstrated by using a blinded instantaneous wave-free ratio (iFR) pullback measurement, which is Philips’ new physiologic guidance technology.
March 20, 2019 — Cook Medical is recalling one lot of its Transseptal Needle due to a manufacturing error that resulted ...


 March 25, 2019
March 25, 2019