Johnson & Johnson Medical Devices Companies announced that Biosense Webster, Inc.’s QDot Micro catheter demonstrated safety and efficacy in achieving pulmonary vein isolation in patients with symptomatic drug-refractory paroxysmal atrial fibrillation (AF).
The Heart Rhythm Society (HRS) 2019 Annual Scientific Sessions represent an important annual event for electrophysiology ...
CardioFocus Inc. announced the presentation of results from its pivotal confirmatory study evaluating the HeartLight X3 System for the treatment of atrial fibrillation (AFib). The results, which demonstrated superior procedural times and impressive procedural outcomes, according to the company, were presented during the Heart Rhythm Society's (HRS) 40th Annual Scientific Sessions, May 8-11, 2019 in San Francisco.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
BioCardia Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the Avance steerable introducer product family, designed for introducing various cardiovascular catheters into the heart, including via the left side of the heart through the interatrial septum.
This is an example of how the heart's left atrial appendage (LAA) can be evaluated for thrombus and possible ...
This is an example of a carotid artery reporting module from Change Healthcare at 2018 Radiological Society of North ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
W. L. Gore & Associates Inc. (Gore) announced the U.S. Food and Drug Administration (FDA) has granted regulatory approval for commercial distribution of the Gore Tag Conformable Thoracic Stent Graft with Active Control System. The device is a thoracic endovascular aortic repair (TEVAR) solution combining new levels of control with the performance of the Conformable Gore Tag Device. The device and delivery system provide new precision and predictable patient outcomes in the endovascular repair of aneurysms, transections and Type B dissections of the descending thoracic aorta. A smaller-diameter primary delivery sleeve gives the device and system a lower profile across 10 device sizes.
Cordis, a Cardinal Health company, recently announced the full U.S. launch of its Radial 360 portfolio, offering a complete range of products to facilitate the transradial approach (TRA) for interventional cardiology procedures.
Artificial intelligence (AI) solutions provider Aidoc has been granted U.S. Food and Drug Administration (FDA) clearance for an additional product in its expanding suite of AI-based workflow orchestration solutions. The clearance is for Aidoc's Pulmonary Embolism (PE) solution that works with radiologists to flag and triage PE cases in chest computed tomography (CT) scans. The approval comes just weeks after Aidoc closed a $27 million funding round, bringing its total funding to $40 million.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

May 15, 2019 — A new study shows electromechanical wave imaging is capable of localizing arrhythmias including atrial ...
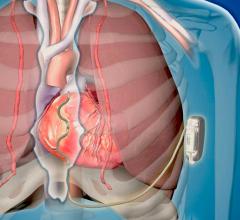
May 15, 2019 — A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The ...
May 15, 2019 — Renal artery denervation (RDN) was found to be safe when employed with pulmonary vein isolation (PVI) to ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
May 15, 2019 - Three new studies show that patients who are medically indicated for implantable heart devices, including ...
May 15, 2019 — A pilot trial has shown His pacing in cardiac resynchronization therapy (CRT) has been shown to ...

May 15, 2019 — The Heart Rhythm Society (HRS) had 21 late-breaking study presentations at the 2019 Heart Rhythm ...


 May 16, 2019
May 16, 2019










![A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The new scoring system was presented as a follow up to that study during a late-breaking session at Heart Rhythm 2019, the Heart Rhythm Society's 40th Annual Scientific Sessions.](/sites/default/files/styles/content_feed_medium/public/PADIT_Infection_Risk_score.jpg?itok=O1-YAcMm)



