May 16, 2019 — CardioFocus Inc. announced the presentation of results from its pivotal confirmatory study evaluating the HeartLight X3 System for the treatment of atrial fibrillation (AFib). The results, which demonstrated superior procedural times and impressive procedural outcomes, according to the company, were presented during the Heart Rhythm Society's (HRS) 40th Annual Scientific Sessions, May 8-11, 2019 in San Francisco.
Petr Neužil, M.D., Ph.D., head of the Department of Cardiology at Na Homolce Hospital (NHH) in Prague, Czech Republic, and the trial's lead investigator, outlined the findings during a presentation titled, "Performance of a 3rd Generation Visually-Guided Laser Balloon for Pulmonary Vein Isolation: Results of the X3 Study." In the evaluation of 60 patients, the HeartLight X3 System consistently achieved very rapid pulmonary vein isolation (PVI), in as few as three minutes for a single vein.
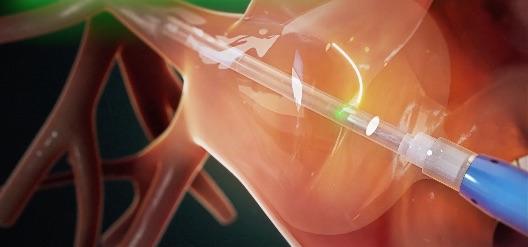
The HeartLight X3 System is a third-generation AFib ablation technology building upon the features of the HeartLight Endoscopic Ablation System, which performs PVI using laser energy to create lines of scar tissue to block the abnormal electrical pathways that cause AFib. Using direct tissue visualization, titratable laser energy and compliant balloon technology, the HeartLight X3 System's RAPID mode leverages a precise motor control system that enables uninterrupted, high-speed, circumferential lesion creation under direct control of the physician. This results in consistently reduced procedure times.
"Our trial results using the HeartLight X3 System were outstanding, with the study meeting all the pre-specified endpoints," said Neužil. "With faster PVI and faster total ablation times combined with the already established safety and efficacy of the current HeartLight System, I anticipate that the HeartLight X3 System will be quickly adopted in the clinical setting."
The key findings of the study included:
-
HeartLight X3 procedure times were more than 90 minutes shorter than with the first-generation HeartLight System[1]; procedures can routinely be completed in one hour [2];
-
The device was able to isolate pulmonary veins in as fast as three minutes;
-
HeartLight X3 demonstrated comparable acute safety and efficacy to the first-generation HeartLight System;
-
Ninety-nine percent of pulmonary veins were isolated acutely with the device; and
-
Fluoroscopy times with HeartLight X3 were under 7 minutes per procedure, substantially lower than with the first-generation HeartLight System
The HeartLight X3 System is only approved for use in Europe. It is not available for sale in the U.S.
For more information: www.cardiofocus.com
References
2 Neužil P., Schmidt B., Chun J., et.al. Performance of a 3rd Generation Visually-Guided Laser Balloon Catheter for Pulmonary Vein Isolation: Results of the X3 Study. HRS 2019. Excluding 30 minute wait period with operators out of the learning curve.


 January 22, 2026
January 22, 2026 









