NorthStar Medical Radioisotopes LLC announced completion of construction on its 20,000-square-foot molybdenum-99 (Mo-99) processing facility in Beloit, Wis., with equipment installation currently underway. Establishing this processing facility is part of NorthStar’s staged development and dual processing pathway approach to expanding current capacity and efficiencies in Mo-99 production.
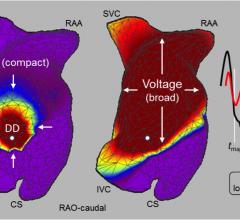
Acutus Medical announced the publication of the UNCOVER AF study in Circulation: Arrhythmia and Electrophysiology. The study demonstrated 73 percent single-procedure freedom from atrial fibrillation (AF) at 12 months with the use of Acutus' AcQMap advanced cardiac imaging and mapping system. Acutus develops electrophysiology (EP) technology solutions built into an open platform EP suite of products that enable personalized and adaptive approaches to therapy.
Edwards LifeSciences is recalling the IntraClude Intra-Aortic Occlusion Device due to a risk of balloon rupture during use, which may add time to the procedure and compromises the safety of the patient.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 15, 2019 — The U.S. Food and Drug Administration (FDA) has approved Gadavist injection for use in cardiac magnetic ...
Internet search engine giant Google unveiled a new Doodle on its homepage Friday, July 12, celebrating the life and work of cardiac surgeon Rene Favaloro. Favarolo, originally from Argentina, was one of the early pioneers of coronary artery bypass graft (CABG) surgery.
This is a 360 degree view of a live cardiac echo demonstration for the Siemens Healthineers Acuson SC2000 cardiovascular ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Mednax Inc. and Mednax Radiology Solutions announced that Chief Medical Officer Ricardo C. Cury, M.D., FSCCT, will present at the 14th Annual Scientific Meeting of the Society of Cardiovascular Computed Tomography (SCCT), July 11-14 in Baltimore. His session is titled “Building a National Centers of Excellence in Coronary Computed Tomography Angiography (CCTA) to Support Implementation in Clinical Practice.”
The Society of Cardiovascular Computed Tomography (SCCT) will present Stephan Achenbach, M.D., FSCCT with the inaugural 2019 Stephan Achenbach Pioneer Award in Cardiovascular CT at the SCCT 14th Annual Scientific Meeting (SCCT2019), July 11 –14 in Baltimore. The award recognizes individuals who have made landmark contributions by demonstrating a commitment to clinical excellence, research and education to the field of cardiovascular CT.
The U.S. Food and Drug Administration (FDA) issued the final guidance “Live Case Presentations During Investigational Device Exemption (IDE) Clinical Trials.”
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

Here is an overview of the hot topics and new technology trends in cardiovascular ultrasound from the 2019 American ...
This is an example of a cardiac echocardiography exam performed using an iPhone and the Butterfly IQ ultrasound ...
This 360 degree photo shows a basic, point-of-care cardiac echocardiogram being performed using a smartphone turned into ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
W. L. Gore & Associates Inc. (Gore) announced the first U.S. implant of the Gore Tag Conformable Thoracic Stent Graft with Active Control System. The successful procedure was performed by William Jordan, M.D., chief of the Division of Vascular Surgery and Bradley Leshnower, M.D., cardiothoracic surgeon, at the Emory University School of Medicine in Atlanta. This first case follows the recent U.S. Food and Drug Administration (FDA) approval for this new device.
This 360 degree photo shows a basic, point-of-care cardiac echocardiogram being performed using a smartphone turned into ...
Neovasc Inc. announced that its Tiara transcatheter mitral valve replacement (TMVR) device and its Neovasc Reducer device for the treatment of refractory angina were highlighted in presentations at the CSI Frankfurt 2019 conference, June 26-29, in Frankfurt, Germany.


 July 16, 2019
July 16, 2019