February 13, 2020 — The U.S. Food and Drug Administration (FDA) has issued its final guidance on "Peripheral Vascular ...
This video illustrates how the Micra AV leadless pacemaker is delivered via catheter and enables atrioventricular (AV) ...
February 13, 2020 — The U.S. Food and Drug Administration (FDA) has approval of Micra AV, the world’s smallest pacemaker ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
February 13, 2020 — The U.S. Food and Drug Administration (FDA) cleared software to assist medical professionals in the ...
February 12. 2020 — Philips announced a new randomized controlled trial to assess patient outcomes after receiving a ...
February 12, 2020 — The U.S. Food and Drug Administration (FDA) has approved Carmat's investigational device exemption ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 12, 2020 — The University of Wisconsin (UW) Health’s University Hospital in Madison, Wis., recently became the ...

Cardiovascular diseases (CVDs) are among the leading causes of death across the globe. For patients suffering from high ...

February 7, 2020 – At the 2019 Radiological Society of North America (RSNA) meeting in December, there was a record ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
February 11, 2020 — An ahead-of-print article in the April issue of the American Journal of Roentgenology (AJR) ...

DAIC Editor Dave Fornell recently took a tour of the Abiomed Impella production line at its headquarters in Danvers ...
Interview with James Udelson, M.D., chief of the division of cardiology at Tufts Medical Center, Boston. The hospital ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
February 7, 2020 — The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the Aria ...
Interview with Navin Kapur, M.D., FAHA, FACC, FSCAI, executive director, The CardioVascular Center for Research and Innovation (CVCRI), director, Acute Mechanical Circulatory Support Program; director, interventional research laboratories; director of Cardiac Biology Research Center, Molecular Cardiology Research Institute (MCRI), Tufts Medical Center. He explains the Door-to-Unloading (DTU) Trial...
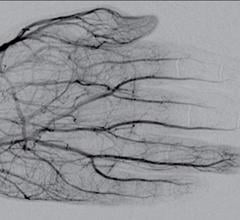
February 4, 2020 — For some patients, kidney cancer can be effectively treated without surgery, according to the Society ...


 February 13, 2020
February 13, 2020