March 13, 2008 - Signalife Inc. received the Frost and Sullivan 'Technology Innovation Award for North American Patient ...
March 14, 2008 - Thirteen of the 18 top-ranked U.S. News and World Report hospitals (72.2 percent) use the noninvasive ...
March 12, 2008 – The Centers for Medicare & Medicaid Services (CMS) today decided not to move forward with its proposed ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Toshiba's fourth generation contrast-free imaging technique, Time and Space Angiography (TSA) creates images that show ...
Biomedical Systems will be featuring its Data Exchange, a proprietary Internet-based application that has been designed ...
This may be the Red Bull of interventional cardiology sessions, since The Society of Cardiovascular Angiography and Interventions (SCAI) and ACC’s Innovation in Intervention: i2 Summit joined forces to form the “SCAI-ACCi2” meeting.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
FUJIFILM’s Synapse 3.2.1 software, an extension of version 3.2, with a new Graphic User Interface (GUI), will be featured at ACC 2008. GUI changes include increase use of color to help identify changes in information on the display, new icons and a contrast scheme designed for radiology viewing conditions.
March 12, 2008 - Siemens will showcase its latest ultrasound technologies, focusing on its ACUSON S2000, ACUSON X300 and ...
A third round of software enhancements made to the iLab System 1.3, an ultrasound imaging system designed to strengthen ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The success or failure of coronary intervention starts with a winning combination of guiding catheter and coronary guidewire, which gives the operator the optimal support and stability to accomplish the interventional goals. The Terumo Runthrough NS coronary guidewire has some unique properties, which allows access to a broad range of complex anatomies.
ScImage’s Web server based Enterprise PACS, PicomEnterprise, employs a single database to organize, categorize, store and distribute exam specific information from multiple departments. This data can be in the form of DICOM and non-DICOM images, clinical reporting, physician dictation, ECG waveforms and even jpeg photos or mpeg videos.
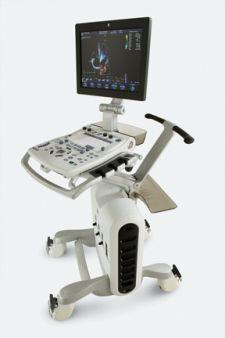
Heart disease is the leading cause of death of Americans, claiming hundreds of thousands of lives yearly. The ability to ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
At ACC 2008, GE Healthcare will be displaying its Innova BiPlane cardiovascular imaging system, one of the first digital flat panel biplane systems with a full-sized lateral plane to cover lateral anatomy without requiring multiple contrast injections and radiation exposures.
Soarian Cardiology is an information solution designed to address the clinical, financial and operational workflow ...

 March 16, 2008
March 16, 2008








