February 24, 2011 – The U.S. Food and Drug Administration (FDA) will no longer require review or approval of technology that helps increase interoperability between devices and information systems. The move is being made to simplify the flow of information between medical devices and electronic medical record systems.
February 24, 2011 – Upsher-Smith Laboratories, is voluntarily recalling one lot (lot #284081) of Jantoven Warfarin Sodium, USP, 3 mg tablets, an anticoagulant with an expiration date of September 2012, NDC # 0832-1214-00.
February 24, 2011 – A percutaneous microwave tissue ablation (pMTA) system will be unveiled at the European Congress of Radiology (ECR) in Vienna, Austria, March 4-7. The Accu2i pMTA system, from Microsulis Medical is cleared for use in Europe, the United States and Canada. Alongside the Accu2i, Microsulis will feature case studies from key centers using the system.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
February 22, 2011 – More than 29 IHE Integration profiles were successfully certified at the IHE North American Connectathon in Chicago. The announcement from Medweb came at the 2011 Healthcare Information and Management Systems Society (HIMMS).
February 21, 2011 – A cardiac computed tomography (CT) report engine was approved by the U.S. Food and Drug Administration (FDA) to save physicians' time, to present instant feedback on their interpretation and to vastly reduce time to diagnosis. The C saves physicians time, presents instant feedback on their interpretation and vastly reduces time to diagnosis.
February 21, 2011 – Scottsdale Healthcare Shea Medical Center in Arizona is the first hospital in the southwestern United States to implant a magnetic resonance imaging (MRI)-safe pacemaker. This represents a major technological breakthrough for patients who need MRI diagnostic scans, which can damage older style pacemakers or cause serious health complications.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
February 21, 2011 – Two drug-eluting coronary stent systems have been launched in India. Boston Scientific’s Promus Element everolimus-eluting stent system and Taxus Element paclitaxel-eluting coronary stent system incorporate the same novel platinum chromium (PtCr) alloy. They also have the same stent design and advanced catheter delivery system.
February 21, 2011 – The first patient has been enrolled the DESSOLVE II study to support CE mark for a coronary stent that uses a bioresorbable drug polymer. The MiStent drug-eluting coronary stent system (MiStent DES), by Micell Technologies.
February 21, 2011 – The U.S. Food and Drug Administration has approved an endoprosthesis device for use on a lower profile delivery system. The Gore Viabahn Endoprosthesis with Heparin Bioactive Surface, from W.L. Gore and Associates, is designed to percutaneously treat peripheral artery disease by relining the native vessel.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

In the world of endovascular devices, the rule is no longer one size fits all. The recent increase in the variety of stents for percutaneous coronary intervention (PCI) offers the physician of a patient with coronary artery disease (CAD) the ability to tailor treatment to the individual, rather than tailor the patient to the therapy.

Recent advances in electrocardiogram (ECG) stress testing systems include better waveform analysis algorithms, improved connectivity with electronic medical records and wireless lead systems to untether patients from the machines. In addition, Web-based software and ECG management systems are making it possible to access ECG stress reports and waveforms from anywhere at any time.
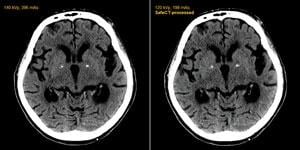
Computed tomography (CT) offers a very important, noninvasive diagnostic tool, but the price of high image quality sometimes comes with a cost of high radiation dose. This is especially true of CT angiography (CTA), which may require imaging several cardiac cycles.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 18, 2011 – A zero-footprint mobile application that enables remote access to imaging tools from hand-held mobile devices, as well as Macintosh- or Windows-based PCs is on display at the Health Information Management Systems Society (HIMSS) 2011 Annual Conference and Exhibition.
February 18, 2011 – The U.S. Food and Drug Administration (FDA) cleared an ultrasound software tool to measure the thickness of the intima-media layers of the carotid artery to evaluate asymptomatic patients' risk of developing cardiovascular disease. Auto-IMT, from Toshiba, is now available on the Aplio XG, Aplio MX and Xario XG ultrasound systems.
February 18, 2011 – An independent study in the journal Circulation confirmed the early and sustained clinical benefits for a system treating aortic valve disease.

 February 24, 2011
February 24, 2011














