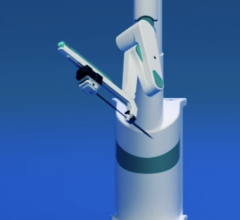
September 20, 2010 - Results for the first-in-human study for a robotic cath lab navigation system will be announced at the Transcatheter Cardiovascular Therapeutics (TCT) 2010 conference. Corindus Vascular Robotics will present information on the study involving its CorPath 200 System.
The study, performed at the Corbic Research Institute in Envigado, Colombia, was conducted on eight patients to evaluate the safety and technical efficacy of the system in delivering and manipulating coronary guidewires and stent/balloon systems in PCI procedures. Juan F. Granada, M.D., medical director, Skirball Center for Cardiovascular Research, Cardiovascular Research Foundation (CRF), was the principal investigator of the study. Juan Andreas Delgado, M.D., and Giora Weiszm, M.D., also participated in the study.
“The trial reached its primary endpoint of zero MACE at 48 hours,” Granada said. “I was impressed by the CorPath 200 System’s ability to precisely control and deliver the guidewire and other angioplasty devices to the target lesion. It definitely helped to perform the procedure in a highly controlled manner. Conducting the procedure while sitting in the system’s cockpit gave me more control and better focus on my patient than I would have achieved standing alongside the procedure table.”
“The procedure time, including setup and patient turnover, was similar to conventional manual operation, which is impressive considering this was the first time we used the CorPath 200 System at my center," Delgado said. All eight patients in the study were discharged with less than 30 percent stenosis following their procedures, and a 30-day follow-up was completed without occurrence of MACE.
According to Weisz, the system represents a paradigm shift in the control of PCI procedures and a major step toward a healthier and safer environment for physicians who spend increasingly longer hours in cath labs.
“The CorPath 200 System allows me to perform a PCI procedure while sitting comfortably in the interventional cockpit without the strain of a heavy lead apron,” Weisz said, adding that his exposure to radiation dropped drastically. “During the study, my mean radiation exposure without a lead apron was only 1.81 µGy. That is 97 percent less than I would have received had I worn an apron and performed the same procedures manually. The system was also very intuitive and easy to use.”
To make an appointment for an individual hands-on demonstration of the CorPath 200 System at TCT 2010, call 1-508-653-3335 x213, or email [email protected].
For more information please visit: www.corindus.com


 January 27, 2026
January 27, 2026