Interventional cardiologists at Mount Sinai Hospital are the first in the world to use a newly U.S. Food and Drug Administration (FDA)-approved device for the treatment of severely calcified coronary arteries before the placement of a cardiac stent to open a blocked artery.

Medtronic Inc. announced the first-in-human implant of the world’s smallest pacemaker: the Micra Transcatheter Pacing System (TPS). The device was implanted in a patient in Linz, Austria as part of the Medtronic global pivotal clinical trial.
TomTec Imaging Systems GmbH announced the U.S. Food and Drug Administration (FDA) 510(k) clearance for its new TomTec-Arena software solution.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
GE Healthcare showcased several new offerings that enable low-dose imaging procedures and help clinicians track and measure dose at the Radiological Society of North America Annual Meeting (RSNA 2013).
Konica Minolta Medical Imaging announced that its parent company, Konica Minolta, Inc., has signed a business transfer agreement with Panasonic Healthcare Co., Ltd. (Panasonic Healthcare) under which the ultrasonic diagnostic equipment business of Panasonic Healthcare is to be transferred to Konica Minolta. All Panasonic Healthcare's assets necessary for planning, development, manufacturing, sales, maintenance, etc., will be transferred, effective on January 1, 2014.

The procedure, first performed in Germany in 2011, has corrected both defects and instilled Brian, a young swimmer and martial arts aficionado, with newfound energy that was in short supply earlier this year.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
With increased penalties in effect for hospitals with excessive readmissions for heart attack and heart failure, the American College of Cardiology (ACC) is launching a program that applies a team approach to keeping patients at home and healthy after discharge.
Konica Minolta Medical Imaging announced the launch of the Sonimage P3 hand-held ultrasound device. The Sonimage P3 brings advanced imaging capabilities to the patient or bedside.
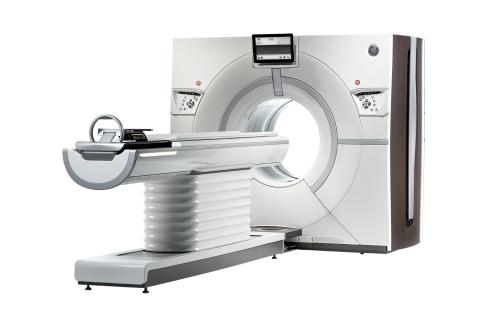
GE Healthcare announced at the Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago that its U.S. Food and Drug Administration (FDA) 510(k)-pending Revolution CT 256-slice system has captured a motion-free image of the human heart in just one beat.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Siemens Healthcare introduced an angiography system optimized for broad clinical utilization at the Radiological Society of North America Annual Meeting (RSNA 2013).
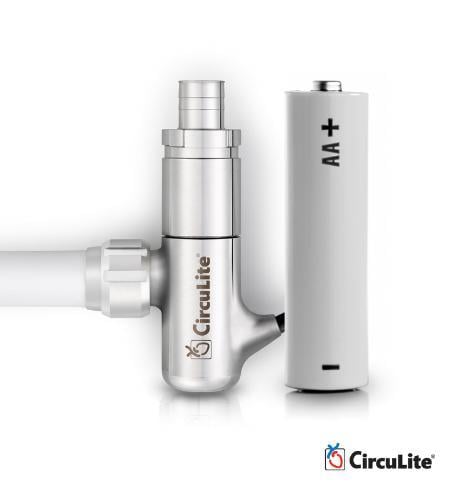
HeartWare International Inc. has acquired CircuLite, Inc., a developer of the Synergy Circulatory Support System, which is designed to treat less sick, ambulatory, chronic heart failure patients who are not yet inotrope-dependent.
Over the past decade, medical imaging has gone from being one of the fastest growing categories of Medicare spending to one of the slowest relative to other Medicare services, according to a new study.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Medtronic Inc. announced CE mark in Europe and Therapeutic Goods Administration (TGA) listing in Australia for its flexible 4 French multi-electrode Symplicity Spyral catheter and Symplicity G3 radio frequency (RF) generator.
Medtronic, Inc. presented three-year data from SYMPLICITY HTN-2, the first and longest-running randomized, controlled clinical trial of renal denervation, which continue to demonstrate results consistent with data reported previously at six, 12 and 24-months of follow-up. The data were presented for the first time during an oral abstract session on Tuesday, October 29, 2013 at the 25th Annual Transcatheter Cardiovascular Therapeutics (TCT) Symposium taking place this week in San Francisco. The Symplicity renal denervation system is available for investigational use only in the United States.
A national group of leading scientists, including one University of Alabama at Birmingham (UAB) expert, said that for more than 100 years, fewer people have been dying of stroke, yet it is still unclear why this decline remains constant.


 December 10, 2013
December 10, 2013