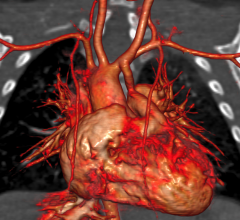
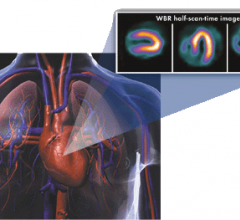
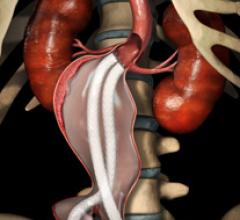
iRhythm Technologies announced that a prospective study by Scripps Translational Science Institute (STSI) has found that use of the company's ZIO Service significantly increased detection of cardiac arrhythmias, compared to use of the traditional Holter monitor.
Biodex Medical Systems Inc. introduced its line of ultrasound tables, which feature redesigned optional side rails that tuck underneath the table when not in use to allow clear access to the tabletop.
A report by MarketsandMarkets analyzes and studies the major technology and market drivers, restraints and opportunities in North America, Europe, Asia and Rest of the World.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Vasomedical Inc. announced that the FDA issued its final order Dec. 30, 2013, reclassifying external counterpulsation (ECP) devices for treatment of chronic stable angina for patients that are refractory to antianginal medical therapy and without options for revascularization from class III to class II (special controls).
The winners of the Right Dose Image Contest were announced at the Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago.
Stentys, developer of the world's first self-apposing stent to treat acute myocardial infarction (AMI), announced new data in indications outside of myocardial infarction.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Barco, a visualization technology company, and Brainlab AG, Germany-based medical software technology manufacturer and marketer, announced their collaboration to provide medical content distribution and sharing.

The annual Radiological Society of North America (RSNA) scientific assembly and annual meeting is where imaging systems vendors showcase their latest technology. While aimed at the radiology market, imaging systems specific to cardiovascular specialties are often highlighted at RSNA first.
DAIC readers chose the following stories as the most popular content in 2013, based on website analytics.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A University of California, Los Angeles research team has found no evidence of an association between iron levels in the body and the risk of atherosclerosis.
As the global medical isotope shortage continues indefinitely following the plant leak in South Africa, Berks Cardiologists Ltd., a cardiovascular imaging center in central Pennsylvania, is continuing to operate at the same procedure volume and with their lower-dose cardiac nuclear medicine imaging protocols.
The American College of Cardiology (ACC) has established the PINNACLE Registry Research Alliance to connect cardiovascular care teams with information about clinical trials and accelerate the systematic access of patients to potentially groundbreaking therapies.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Endologix Inc. announced that it has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to begin a clinical trial to evaluate the safety and effectiveness of the Nellix EndoVascular Aneurysm Sealing System (EVAS).

St. Jude Medical Inc. received European CE mark approval for its 25-mm Portico Transcatheter Aortic Heart Valve Implantation System.
Medtronic Inc. announced that the first patients were randomized in Symplicity HTN-4, which will evaluate the Symplicity renal denervation system in patients with moderate uncontrolled hypertension.

 January 08, 2014
January 08, 2014