The ScottCare Corp. announced its new novi patch Holter monitor will be available in the United States for the first time at the 2015 Scientific Sessions of the American College of Cardiology in San Diego, Calif., March 14-16.
CBSET, a not-for-profit preclinical research institute, announced that its scientists have defined the optimal tissue debulking protocol for treating in-stent restenosis (ISR) with Boston Scientific’s Jetstream Navitus atherectomy device. These findings were disclosed at the Cardiovascular Research Technologies 2015 annual scientific meeting (CRT 2015) in Washington, D.C.
Sunshine Heart Inc. announced it has received unconditional approval from the U.S. Food and Drug Administration (FDA) to conduct an interim analysis of COUNTER HF, the company's U.S. pivotal study.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
SuperSonic Imagine announced a partnership with U.S.-based Unetixs Vascular Inc. to bring SuperSonic Imagine’s technology to more customers in the vascular market.
In light of a recent American Heart Association report stating that U.S. mitral valve regurgitation prevalence was 1.7 percent in 2014, a significant opportunity exists for development of an effective transcatheter mitral valve replacement (TMVR) device as an alternative treatment to surgery for inoperable patients. This assessment was given by an analyst with research and consulting firm GlobalData.
Daiichi Sankyo and the Heart Rhythm Society announced results from a global survey, which polled cardiologists from around the world and revealed that a majority (58 percent) agree there is no such thing as a "typical" non-valvular atrial fibrillation (NVAF) patient.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The National Forum for Heart Disease & Stroke Prevention announced the launch of the Stronger Hearts Helpline, a 24/7 free call-center resource for people with heart failure and their families. The pilot program is an addition to the existing 2-1-1 information and referral hotline in San Bernardino County, California – a region where nearly 20 percent of Medicare recipients are being treated for heart failure – and long-term plans are to expand the service nationwide.
Americans are ready and willing to leverage health apps and wearable devices to improve their personal health, according to the findings released from the fifth annual Makovsky/Kelton "Pulse of Online Health" Survey.
Medtronic announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has approved a transitional pass-through payment for the company’s IN.PACT Admiral drug-coated balloon (DCB) under the Medicare hospital outpatient prospective payment system (OPPS). The decision removes a potential barrier to patient access to this new medical device, which represents an improvement to the standard of care for peripheral arterial disease in the upper leg.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
C. R. Bard Inc. announced that the U.S. Centers for Medicare and Medicaid Services (CMS) has approved a pass-through payment for the Lutonix drug-coated balloon (DCB) under the Medicare hospital outpatient prospective payment system. This approval follows a unanimous favorable recommendation from the U.S. Food and Drug Administration’s (FDA) Circulatory Systems Devices Advisory Panel.
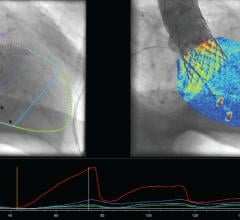
Pie Medical Imaging BV announced that it received 510(k) clearance from the U.S. Food and Drug Administration for its CAAS A-Valve product including the quantitative Regurgitation Analysis (qRA) workflow. The qRA workflow is the first 510(k) cleared image analysis technology to determine aortic regurgitation based on X-ray angiography.
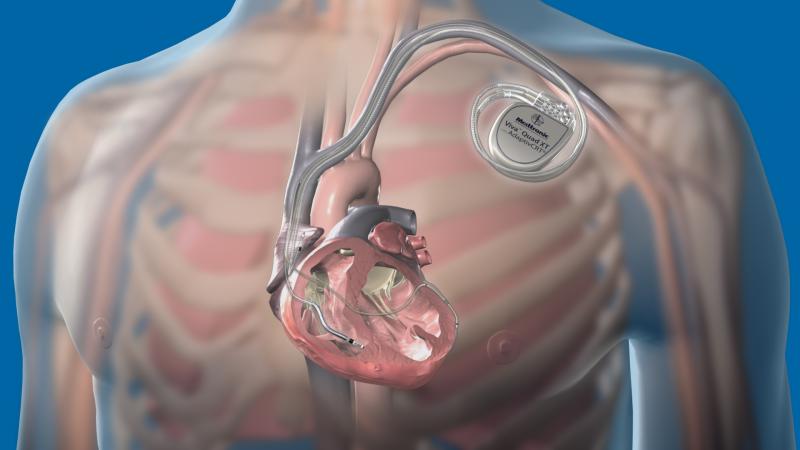
There have been several recent advancements in implantable cardioverter defibrillator (ICD) technology to extend battery life, improvements in patient monitoring to avoid needless shocks, the introduction of quadripolar lead devices to improve device programming and to improve therapy effectiveness, and development of MRI-safe ICDs.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Agfa Healthcare announced the commercial launch of its Enterprise Imaging for Cardiology Suite and strategic relationship with TomTec to provide a unified cardiology diagnostic and clinical imaging solution. The solution will be on display at the American College of Cardiology (ACC) 2015 meeting, March 14-16, 2015 in San Diego.

Medtronic announced the results of a new "real-world" study of patients who have had a cryptogenic stroke, in which the Reveal Linq Insertable Cardiac Monitor (ICM) detected atrial fibrillation (AF) in everyday practice at an even greater rate than that found in a recent clinical trial (the CRYSTAL AF Study, which was published in The New England Journal of Medicine).
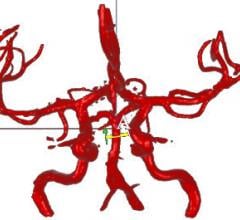
A recently completed National Institutes of Health-funded trial confirms the efficacy of blood flow measurement technology from VasSol Inc. to predict the possibility of repeated stroke in at-risk individuals.


 February 25, 2015
February 25, 2015